Arrhythmia means abnormal heart rate or rhythm; too fast (tachycardia with rate >100 bpm), too slow (bradycardia <50 bpm) and irregular (extra beats, dropped beats, atrial fibrillation/ flutter).
Palpitation describes feeling the heart’s beat, usually stronger than usual, and arrhythmia is not always the cause.
Arrhythmias include innocent premature beats that may be present in a low number in most, even healthy subjects. If they appear in a large number, they may suggest the presence of cardiac disease, and be a warning signal. If the heart stops beating for a few seconds, dizziness or syncope may result, and a prolonged arrest may be fatal. Further, there are paroxysmal tachycardias that are bothersome and occasionally disabling, and there are potentially life-threatening episodes. Fortunately, several treatment options are available: antiarrhythmic drugs, catheter ablation that destroys the arrhythmogenic site or mechanism, pacemakers and implantable cardiac defibrillators. For the general practitioner the main challenge is to discern dangerous arrhythmias from the innocent ones, since the treatment of arrhythmias usually is guided by a specialist in internal medicine or cardiology.
The most important diagnostic tool for arrhythmias is the history, plus pulse recording and ECG:
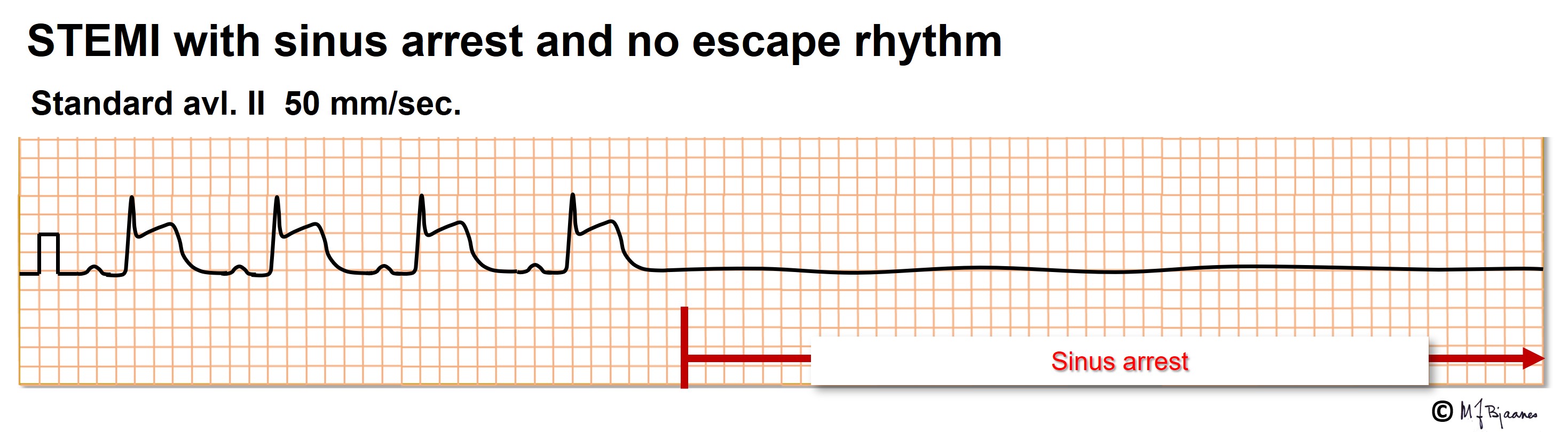
The normal heart beat starts in the sinus node that contains the fastest pacemaker cells. If no impulse should reach the atrium, and no atrial rescue impulse appears, there will be a pause without any P wave. The pause – sinus arrest - may be brief or long. When a pause exceeds 3-5 seconds, the patient may experience dizziness or a fainting spell. Usually, the normal rhythm soon recovers, and the patient immediately feels well.
In well-trained athletes the vagal nerve is very active. The resting heart rate is low, and during sleep, a few seconds of sinus arrest are common. They are not noticed, do not indicate bad health, and even severe bradycardia does not require pacemaker treatment.

In some patients the vagal nerve is too sensitive and active, and this can be demonstrated by pressure on the site where the internal and external carotid artery divide (see a textbook). Examination of a syncopal patient aged >40, should include such testing during ECG recording. The test may induce sinus arrest, AV block and/or a primary fall in blood pressure (fig below). When bradycardia or pauses dominate, treatment with a pacemaker may be recommended.
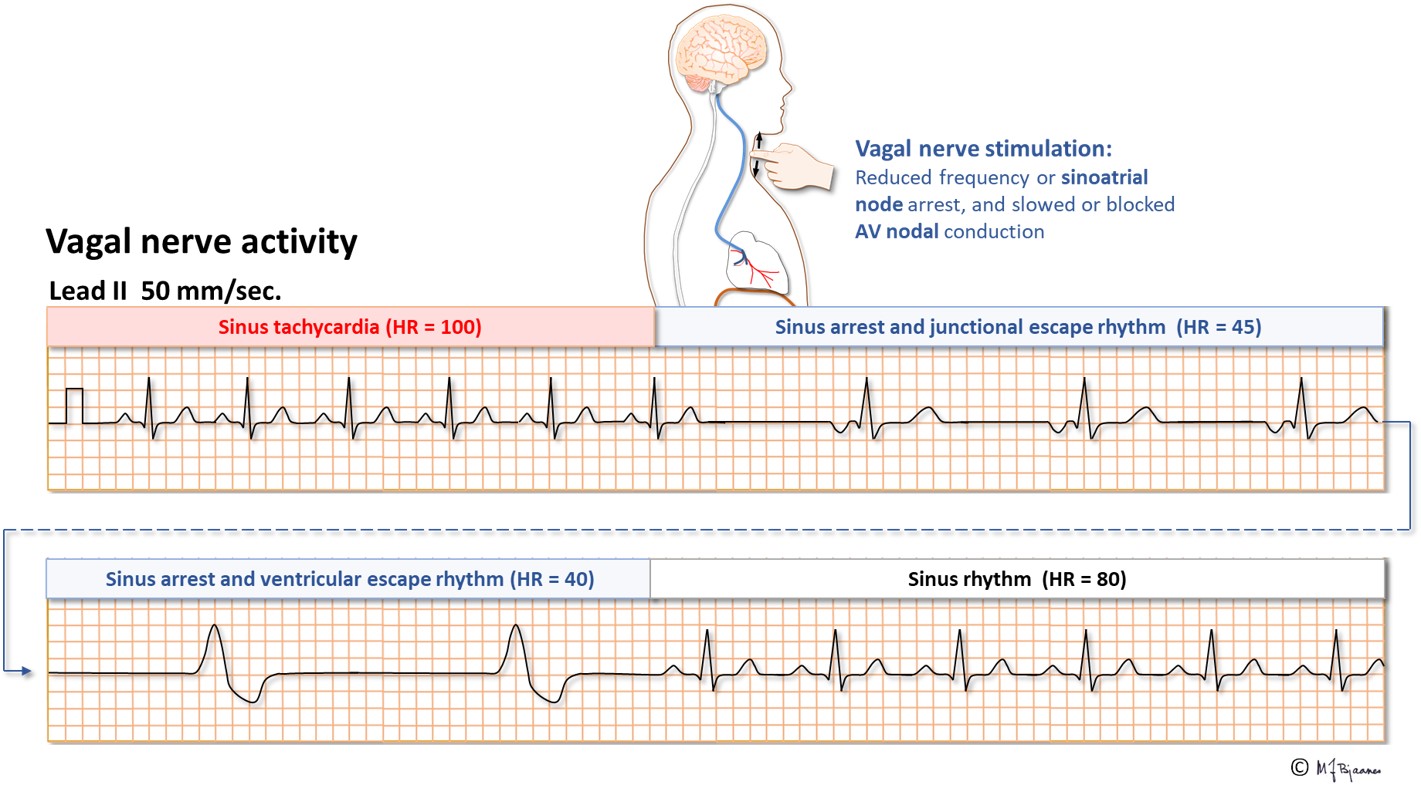
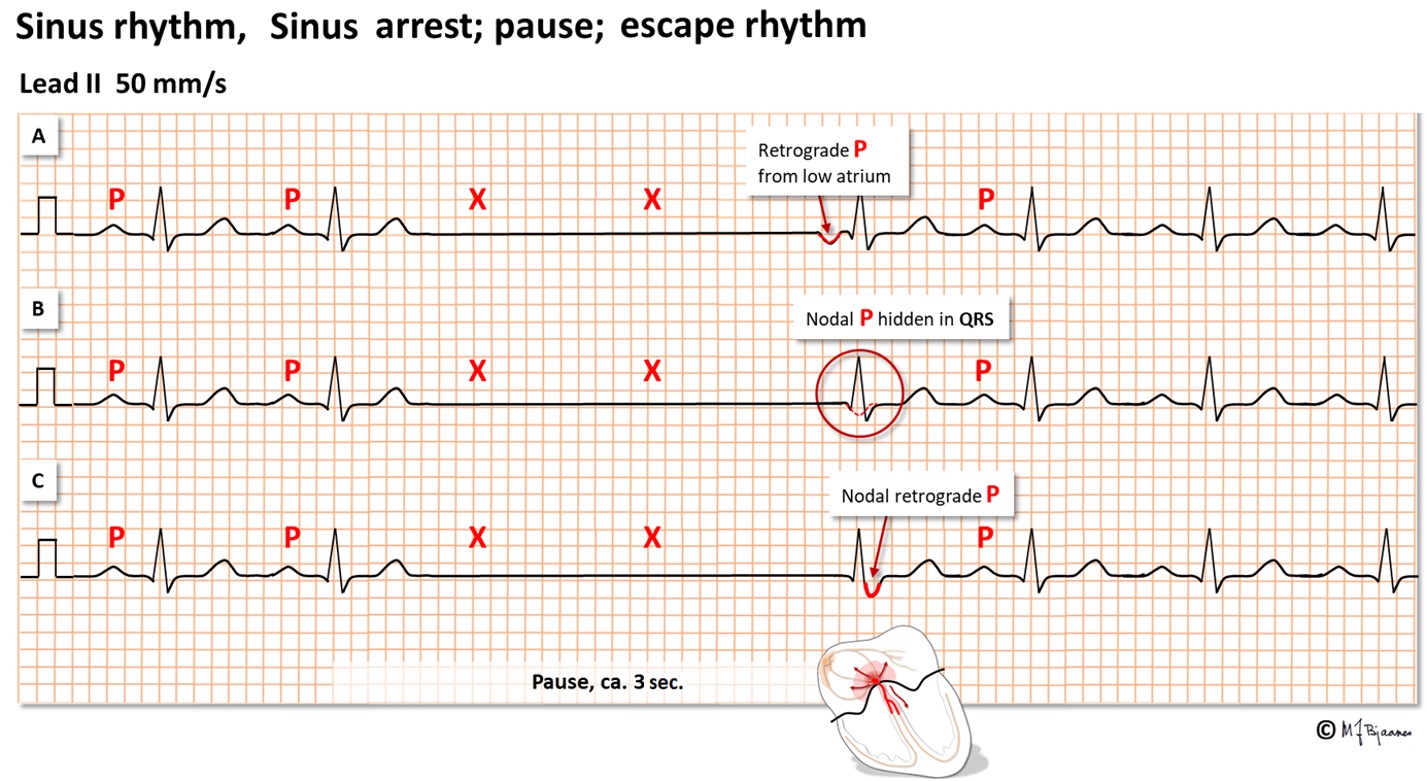
Below, three forms of escape beats are shown. When the sinus node fails, there is sinus arrest. The situation is rescued by the AV node that generates an impulse that spreads down the conduction system and gives a normal QRS. The AV nodal impulse will also spread upward, resulting in a retrograde P wave (negative in II, aVF and III) that activates the atria simultaneous to ventricle depolarization. This P wave can be seen either just in front of or just after the QRS, or it is hidden within the QRS complex. Finally, the normal sinus rhythm recovers. The sinus node is long and crescent shaped, and the lower part may generate a slow escape rhythm when needed.
Sinus arrest postpones the spontaneous pacemaker activity of the sinus node for a while, creating a P wave pause. Occasionally, the sinus node cells depolarize normally, but the impulses are blocked and never reach the atrium. Hence, no P wave will be seen. This sino-atrial block (SA block) is characterized by a pause in P waves that lasts for an exact multiplum of normal P to P intervals, and after the pause, the unchanged sinus rhythm continues.
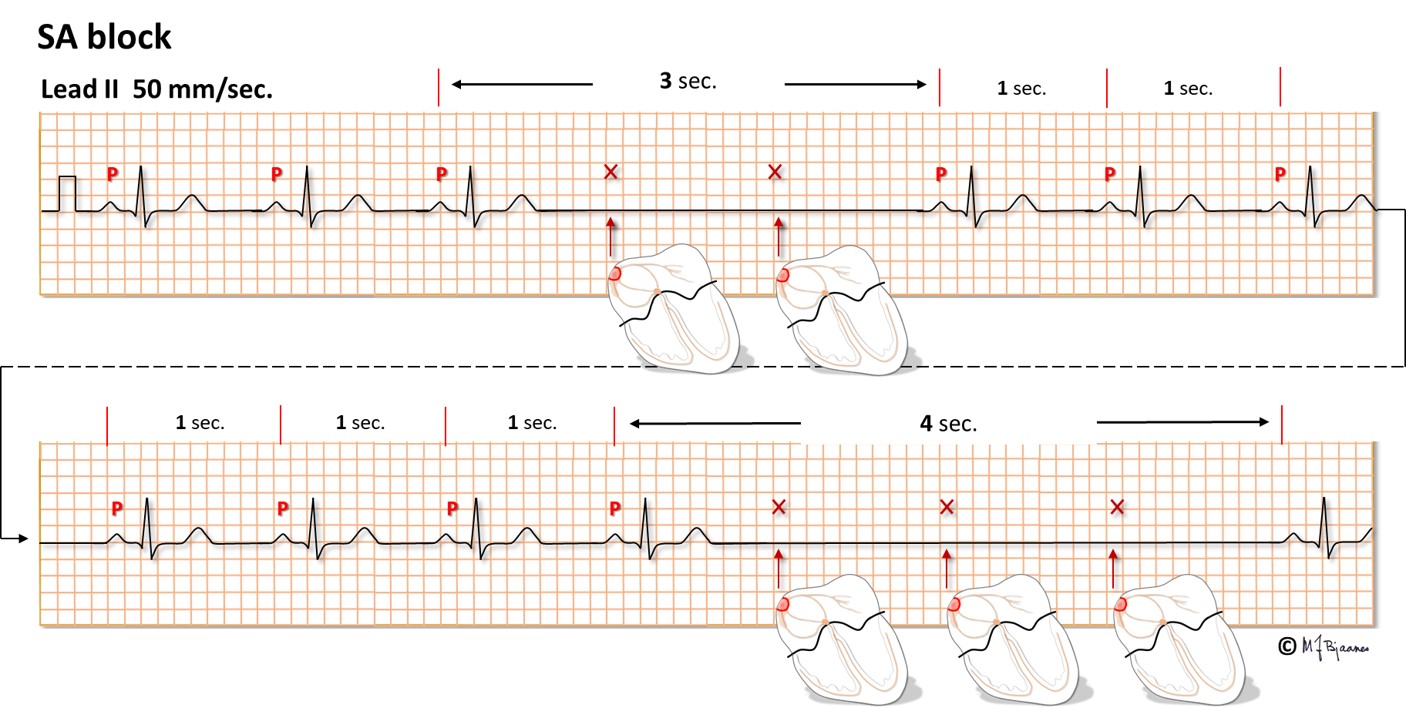
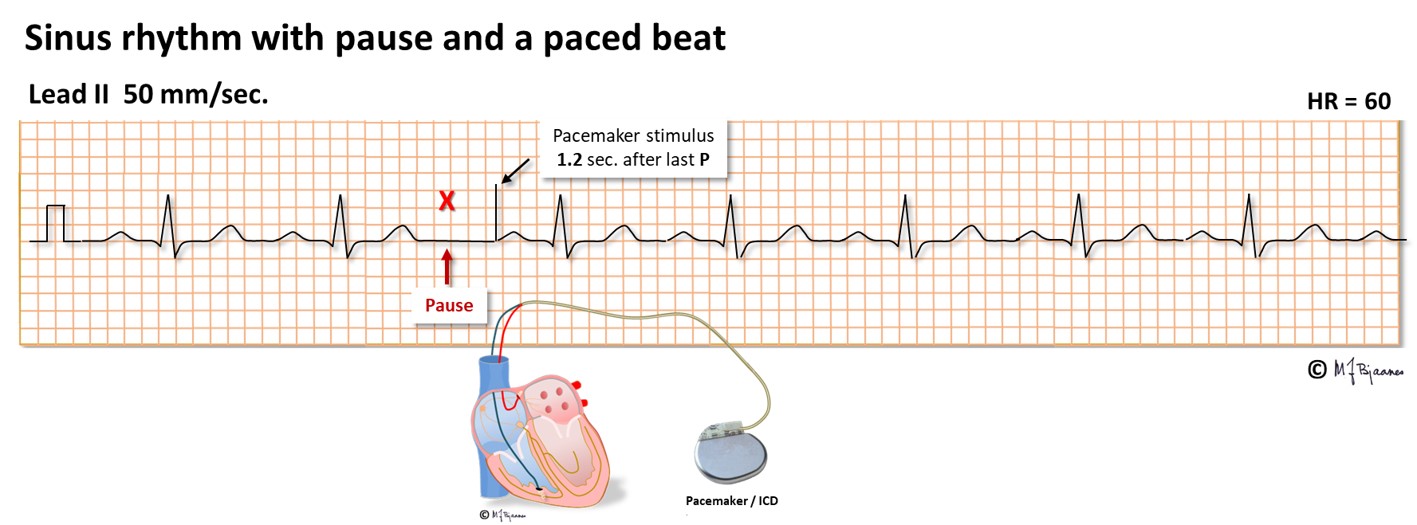
We call it a sick sinus syndrome (SSS) when a patient has symptomatic atrial pauses due to sinus arrest or exit block (SA block), and such patients may be treated by a pacemaker. The tip of the pacemaker lead is screwed into the right atrial wall, and each spontaneous P wave resets the timer of the pacemaker. If a spontaneous P wave does not appear, the pacemaker stimulates the atrium after a programmed interval (notice the spike in front of the P wave below). When spontaneous P waves reappear, the pacemaker goes to rest.
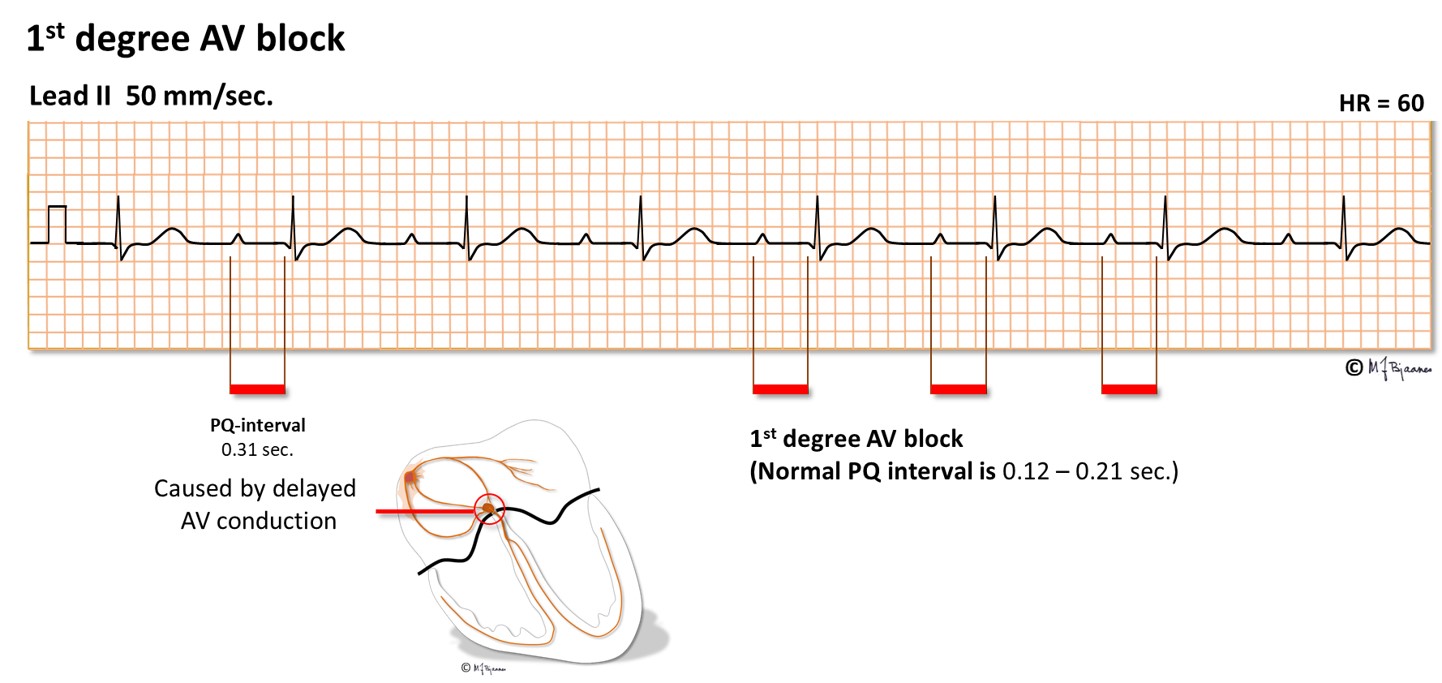
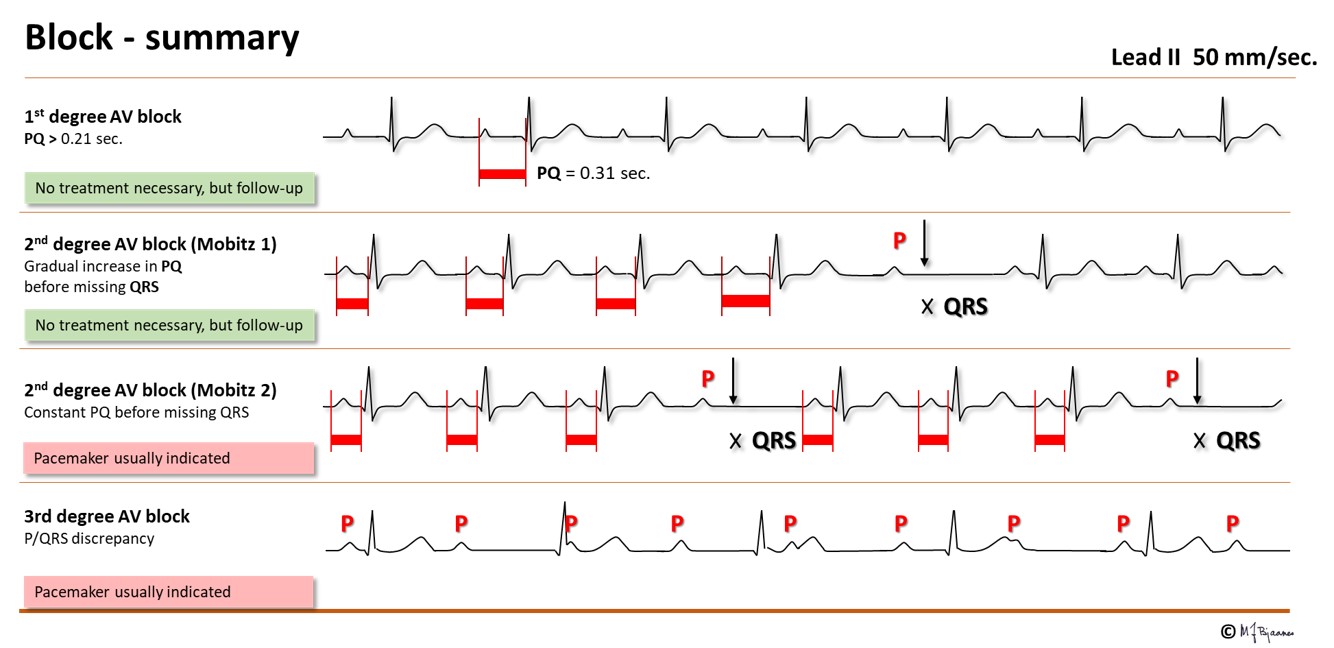
The conduction time from the atria to the ventricles is measured from P wave onset to start of the ventricular complex (the Q wave, or if none, the R wave onset). The PQ (PR) interval comprises the first atrial depolarization, the impulse conduction across the atria, the AV nodal delay and the conduction through His’ bundle and the branches until the first visible sign of ventricular activation. AV nodal delay is the main contributor to the PQ (PR) interval. The normal interval is 0.12-0.21 s, but in case of AV nodal injury or strong vagal activity, PQ (PR) interval will be prolonged. When every P wave is conducted, but the interval is prolonged (0.22+ s), we call it a first degree AV block. A first degree AV block with a PQ (PR) interval up to 0.30 s is usually unnoticed by the patient and needs no treatment. However, if the delay is so marked that the atrial contraction occurs efore the end of the previous systole, the atrial pressure wave cannot open the closed AV valves, and the pressure is transmitted backward, giving discomfort in the chest (from left atrium) or on the neck (from right atrium). Athletes frequently have a moderate first degree AV block that is accepted as normal.
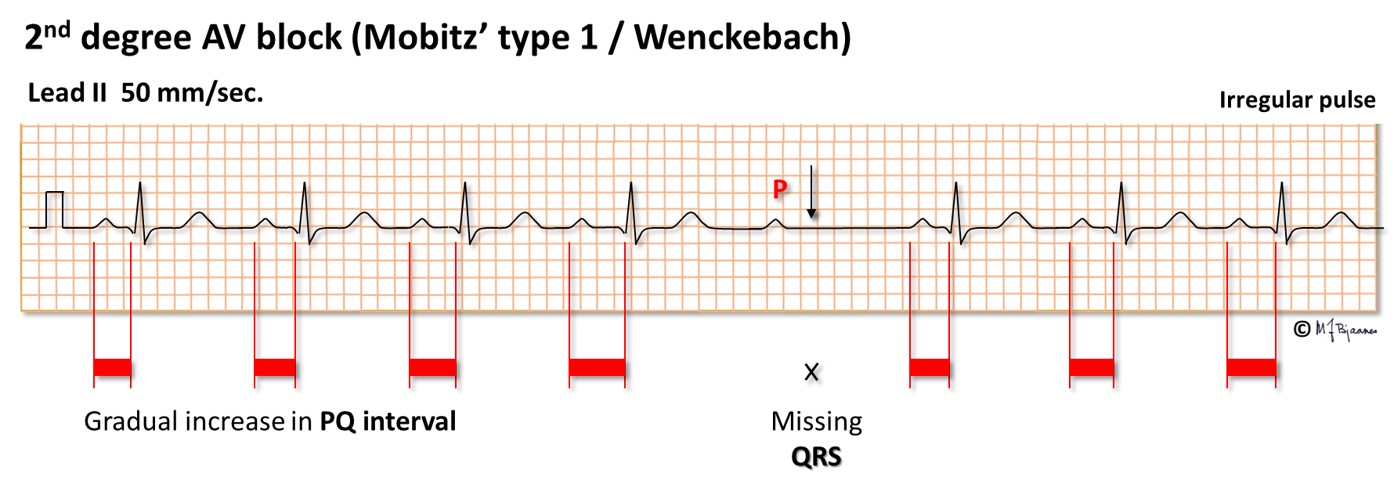
In a 2nd degree AV block one QRS complex is dropped, whereas the preceding and following P waves are conducted. Usually patients will not notice this, or they just have a brief strange feeling in the chest (a palpitation).
The common and innocent form of 2nd degree AV block has a characteristic pattern: the first PQ interval in the sequence is normal, the next slightly prolonged, the next even more, until a QRS is non-conducted, and the sequence repeats (illustrated below). This is called Mobitz’ type I block (and also Wenckebach block), and is neither associated with disease nor syncope. Athletes often have such AV block during sleep.
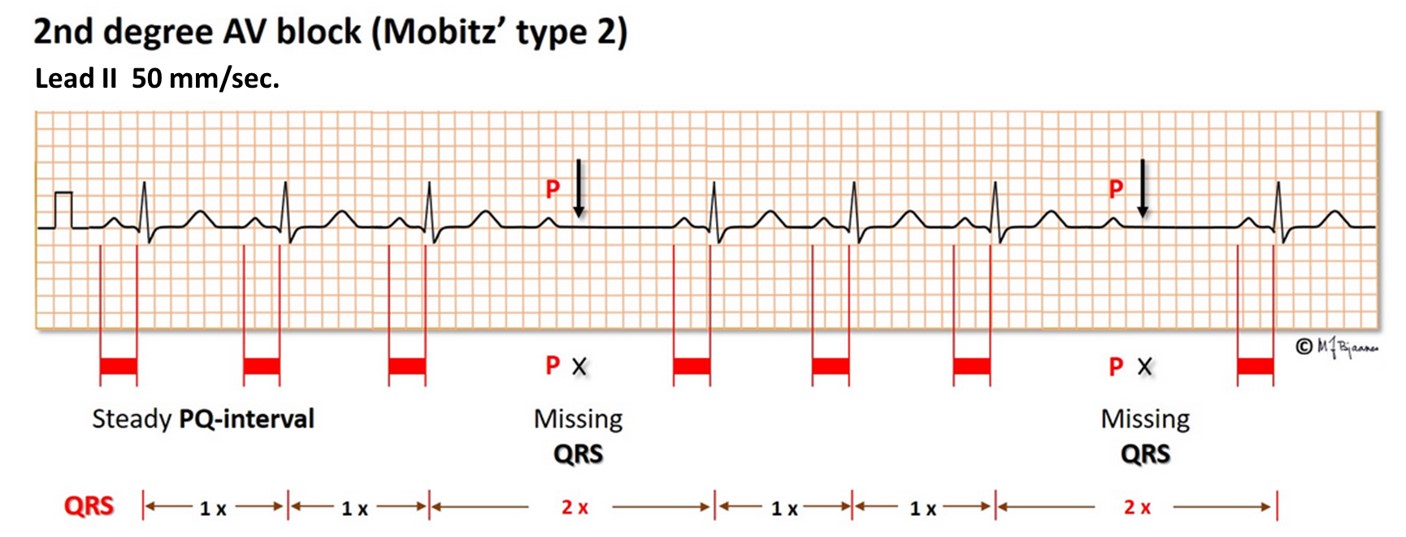
In Mobitz’ type 2 second degree AV block a random QRS is dropped. This usually indicates a severe conduction problem, and is not “permitted” in a healthy athlete. Actually, implantation of a pacemaker may be indicated even in a symptom free patient to prevent syncope.
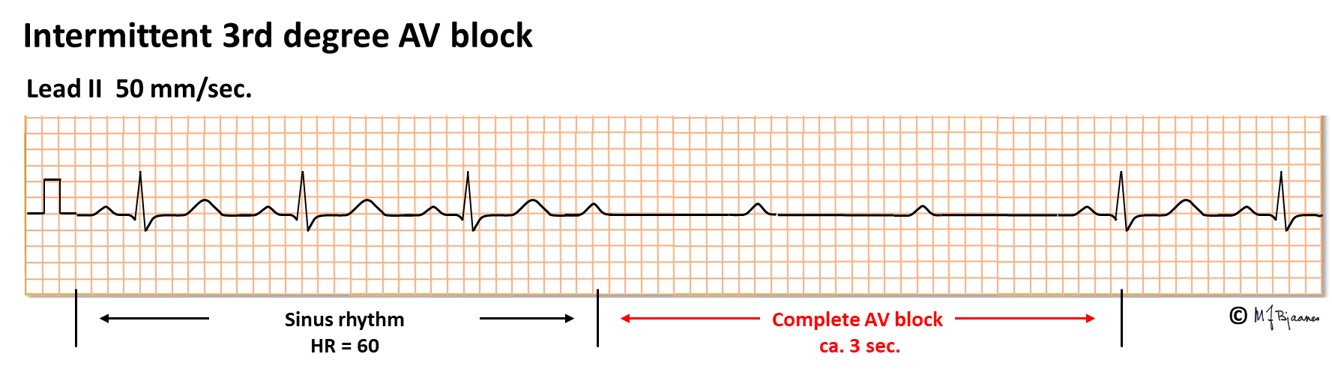
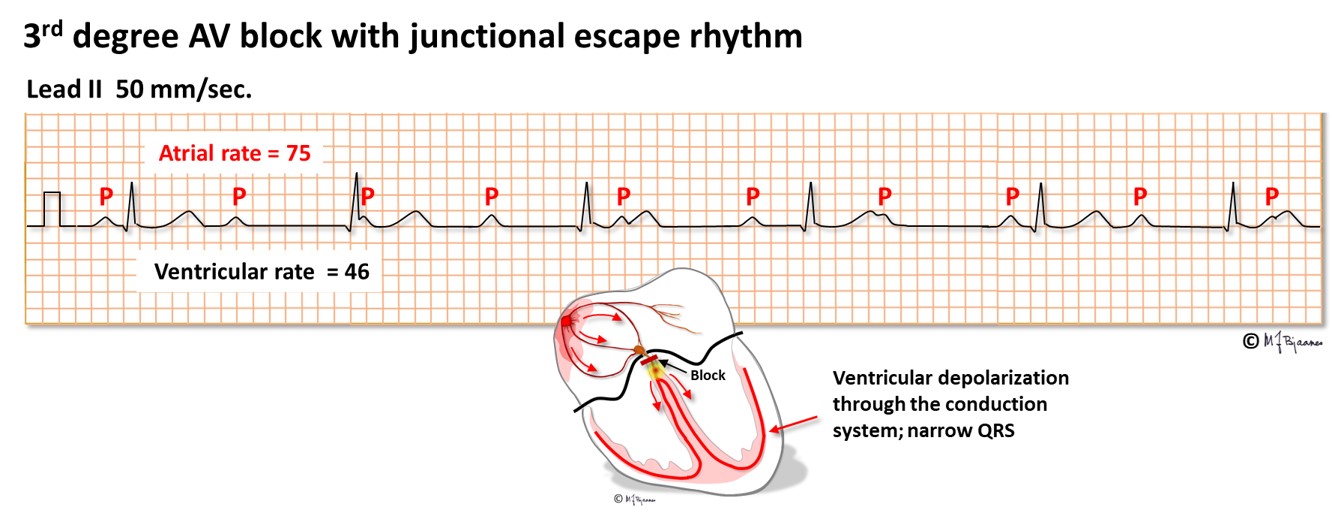
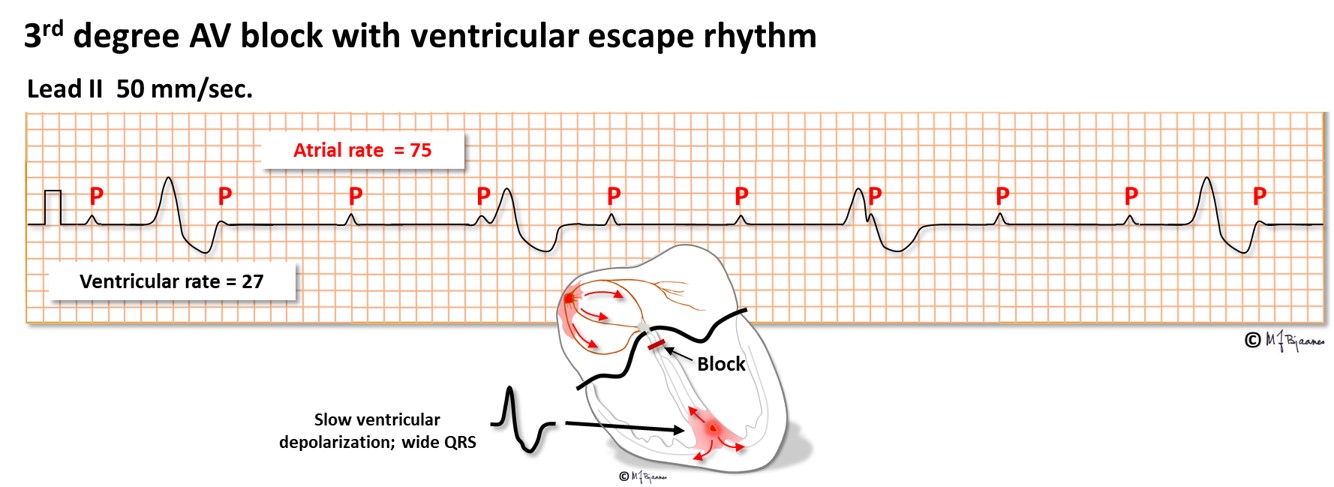
AV block of third degree is a complete heart block, resulting in independent atrial and ventricular rhythm. Either the AV node or the His’ bundle fails to conduct (or theoretically, the right bundle + the two left-sided fascicles). The escape rhythm is generated from low in the AV node or His’ bundle (narrow QRS complexes) or from further down (with broad QRS). Third degree AV block may be permanent, usually with a slow escape rhythm that results in heart failure with time, or intermittent with brief or longer periods of heart arrest. Without rescue from an escape rhythm the patient will faint, or even die. Syncopal spells caused by transient AV block are called Stokes-Adams attacks. If a 3rd degree AV block is found without a correctable cause, the patient needs an implanted pacemaker. A high degree conduction block usually signifies disease in the entire conduction system.
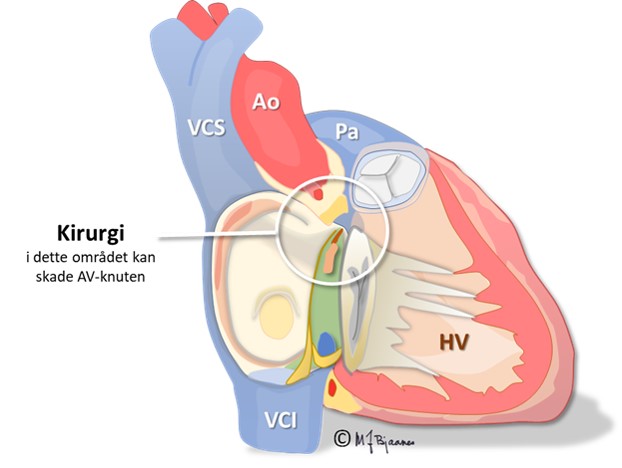
Surgery, inflammation/infection or injury in the «center of the heart» may result in AV block.
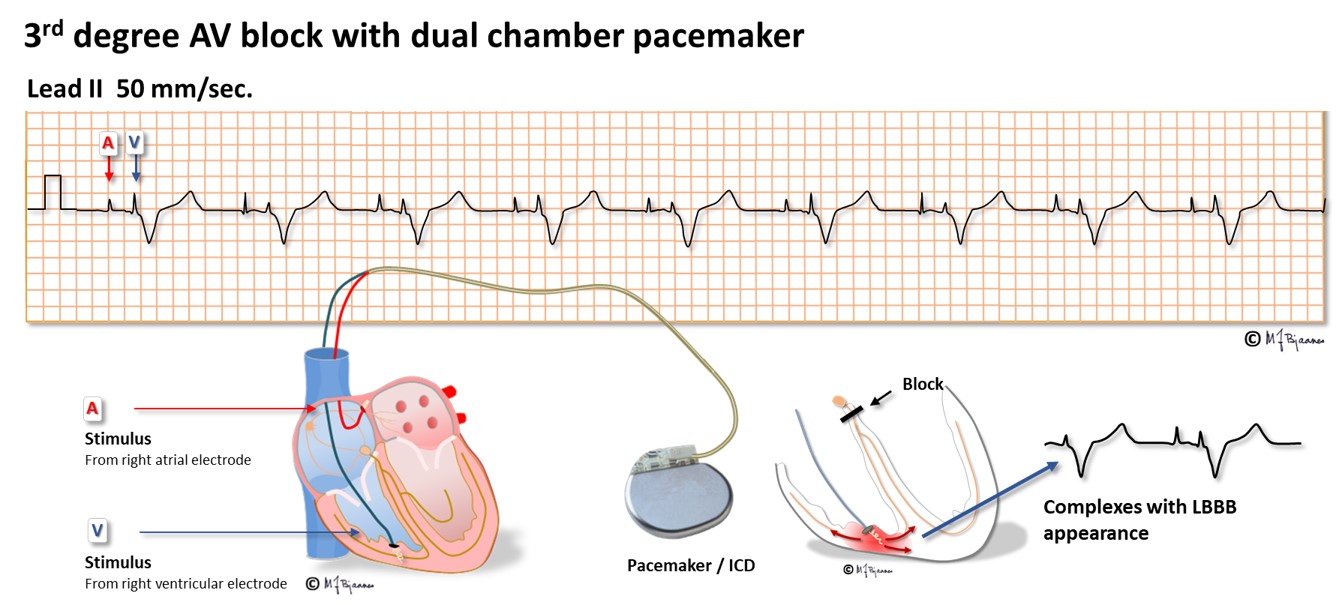
Rarely a pacemaker is implanted to relieve symptoms from a 1st degree AV block with a long conduction time, and the majority of pacemakers are implanted for 2nd degree AV block with syncope or 3rd degree block. The pacemaker senses the P waves through the atrial lead and stimulates through the ventricular lead, with an appropriate AV delay (see below):
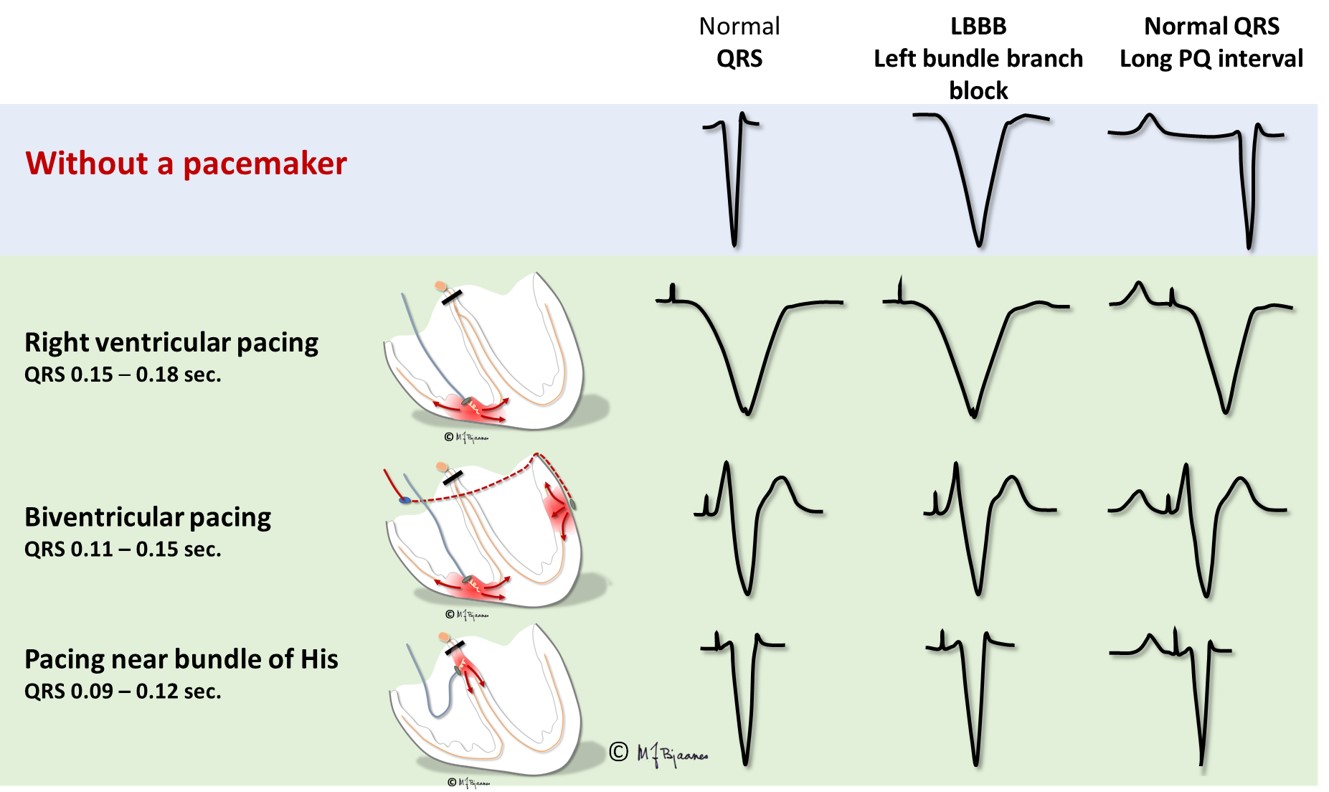
Common ventricular pacing stimulates the right ventricle near apex, through an electrode with screw-in fixation into the myocard. From the tip of the lead depolarization waves propagate through myocard, first outside the conduction system until it is penetrated and conduction speeds up. The QRS is broad, usually 0.15-0.16 s. As the right ventricle is stimulated, the QRS complexes will look like a left bundle branch block. Such a pacing mode is tolerated by the otherwise healthy heart, but the induced dyssyncrony of the right and left ventricle results in impaired emptying of the ventricles and worsening of a heart failure, if present. If another lead is placed in a coronary vein on the left ventricle, simultaneous stimulation of the two ventricular leads may resynchronize the ventricles, reduce QRS width and improve the pump function. For a block in the AV node, an electrode that is fixated into nearby septum may provide biventricular stimulation with close to normal QRS complexes.
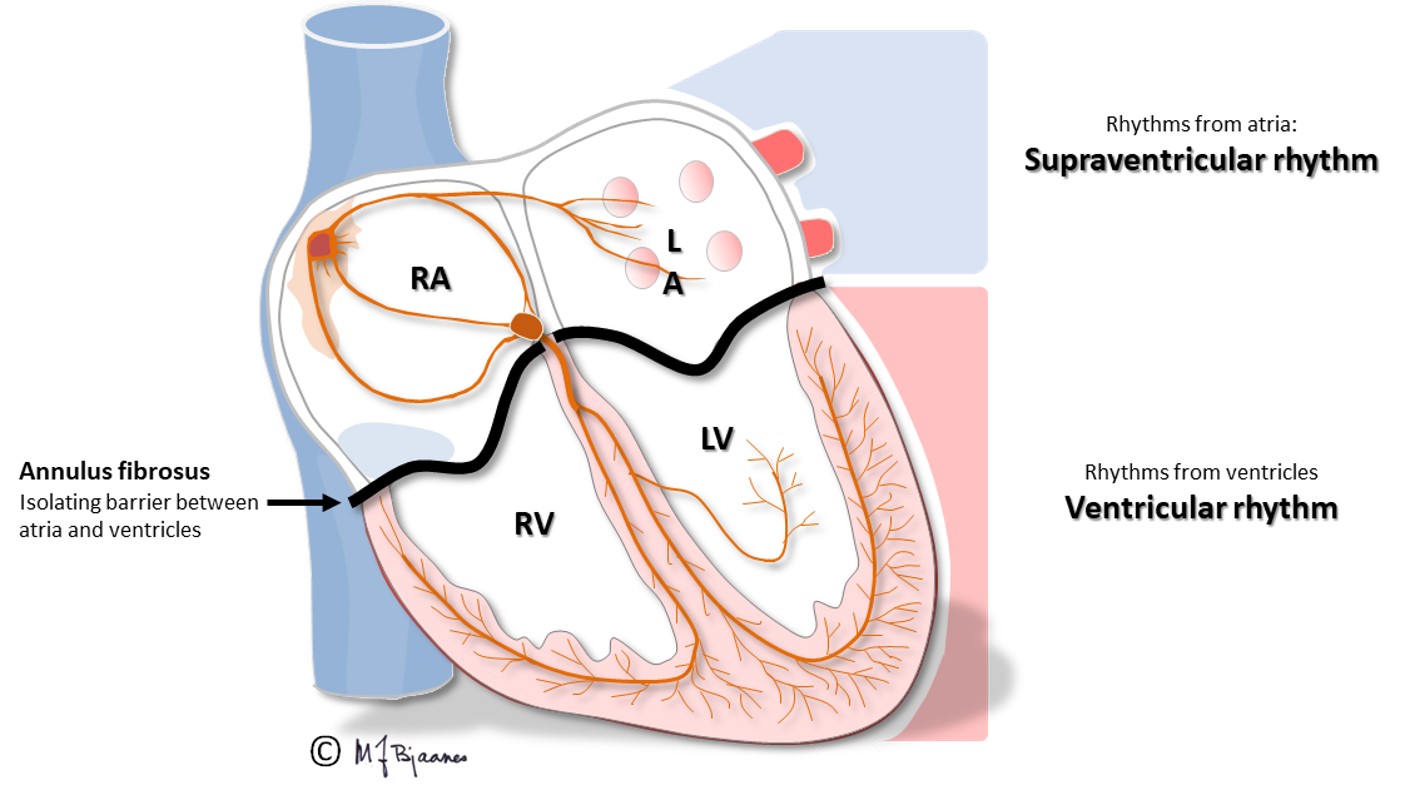
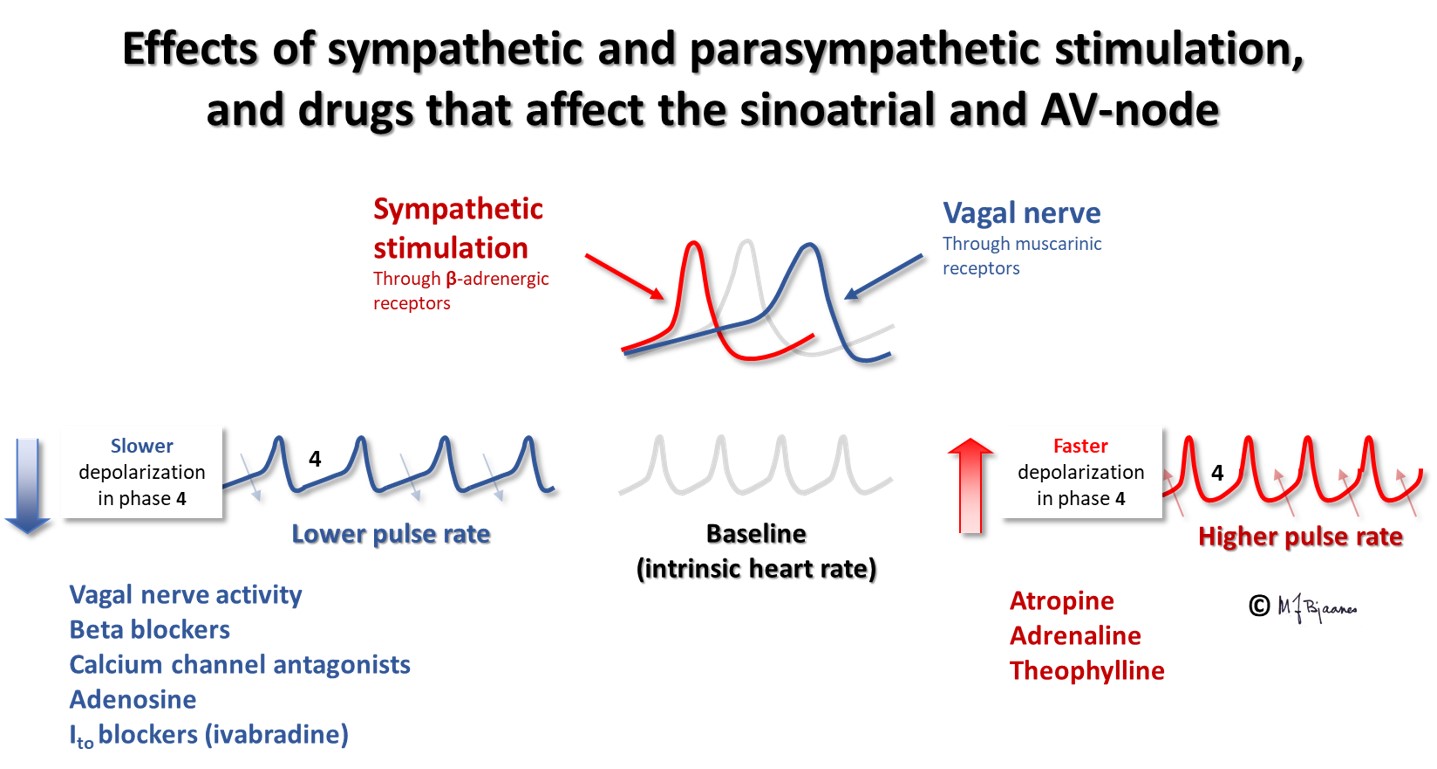
The sinus node is richly innervated by both vagal and sympathetic nerve fibers. Vagus is activated by expiration, and the heart rate slows down. Atrial wall stress during filling deactivates the vagus nerve, and heart rate speeds up during inspiration, as the low intrathoracic pressure sucks venous blood into the right atrium. Such respiratory rate variations are most marked in children and the young, more in well trained than in sedentary persons, and this heart rate variability (HRV) is reduced after a myocardial infarct, in heart failure and other diseases. HRV is described by statistical methods.
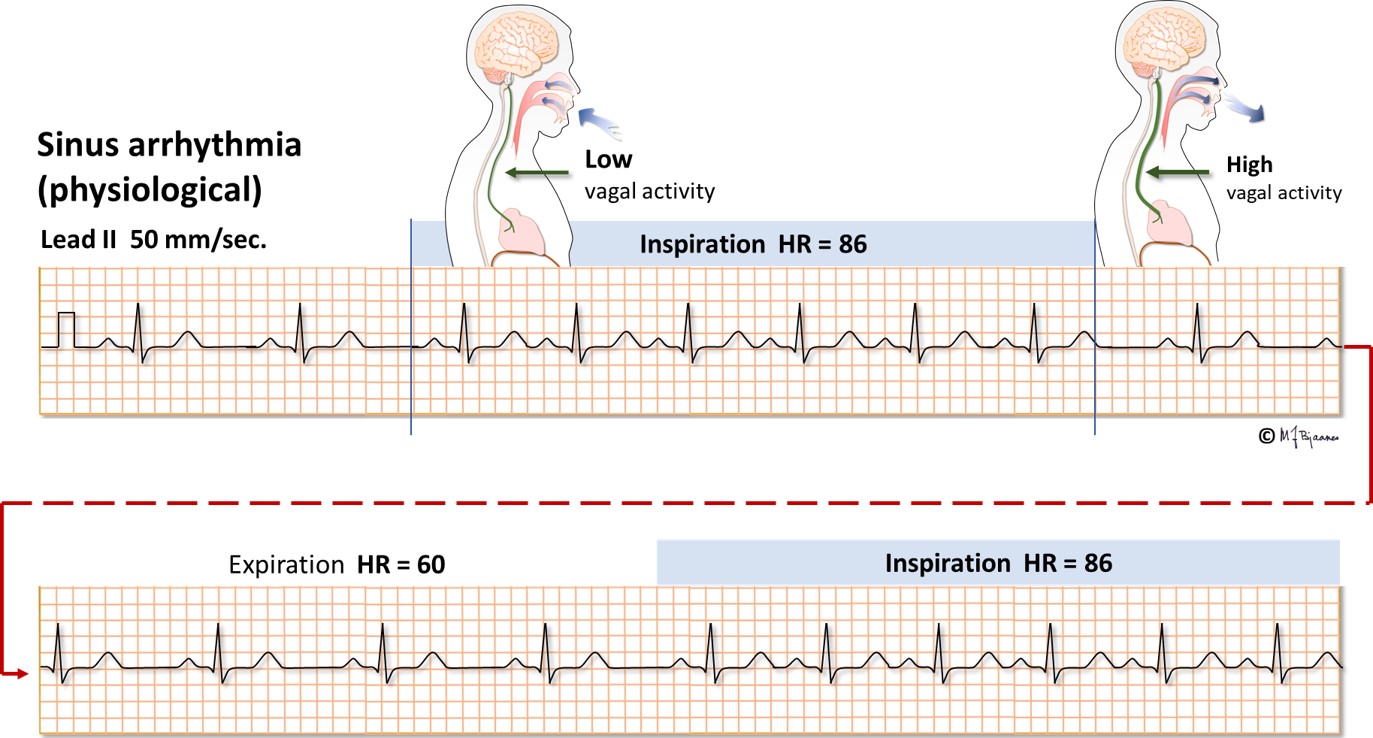
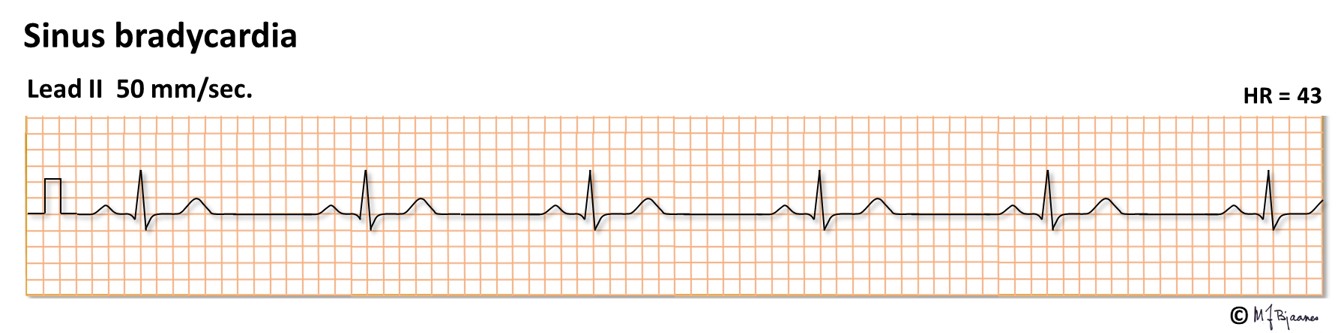
Sinus rhythm <50 bpm (previously <60) defines sinus bradycardia. This is common among the well trained, and is associated with good health. Improper bradycardia may reflect a «sick sinus syndrome» or a drug effect (for instance beta-adrenergic blockade).
In adults normal sinus rhythm is 50-100 bpm. In tachycardia the heart rate is faster. In sinus tachycardia the P waves are generated by the sinus node (high up in right atrium), and some respiratory rate variation should be seen, and the axis should point downward to the left (positive in leads I and aVF). If the resting heart rate is >100 bpm, an explanation should be sought for. In tachycardia the systole (QT interval) is shortened, but more so, the diastole shrinks (short T-Q interval), and the P wave comes closer to the preceding T wave. The two may even fuse.

Sinus tachycardia may have various causes:
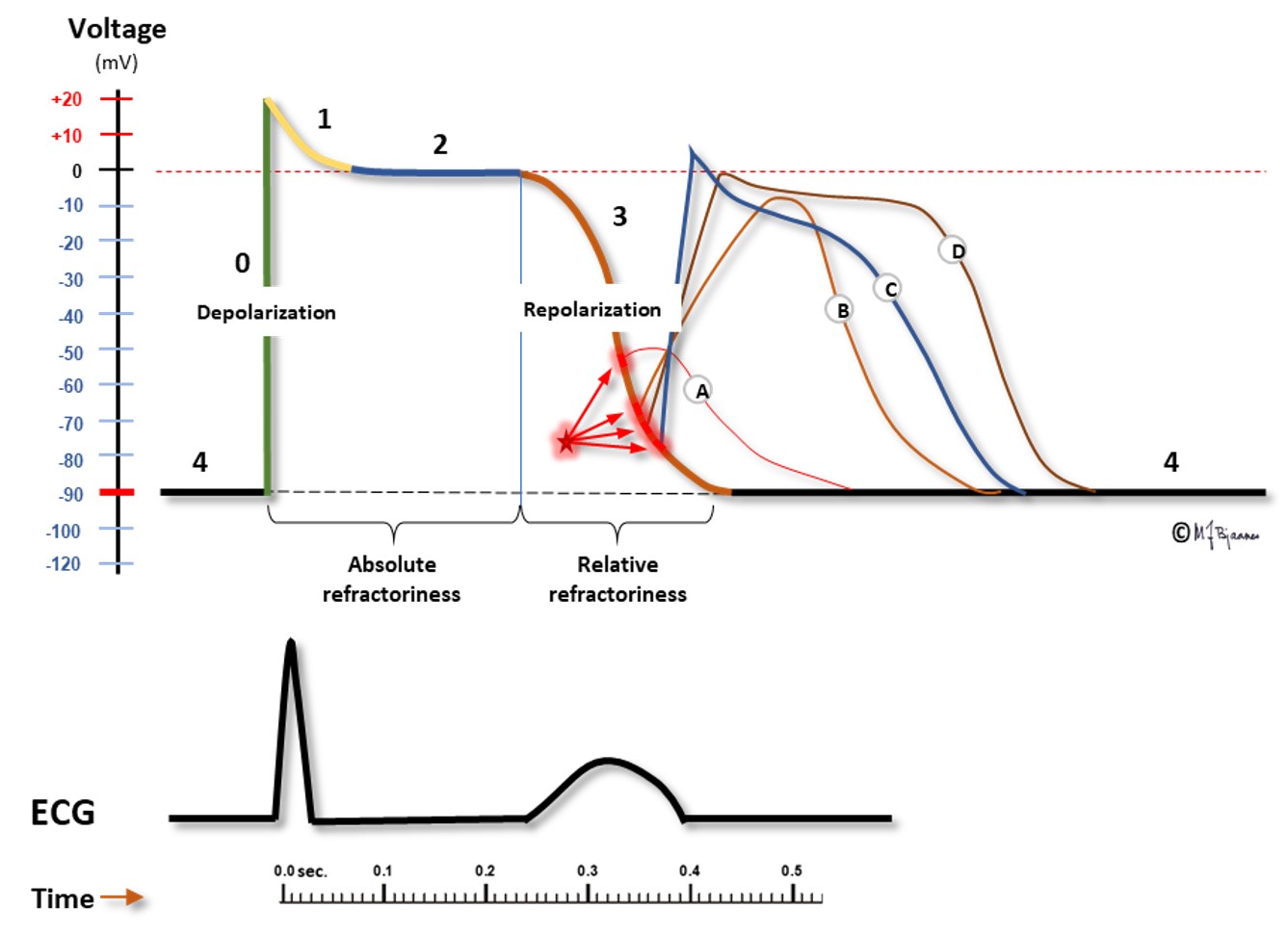
An atrial extrasystole is premature, and may differ from the usual P wave morphology when its origin is outside the sinus node. An early depolarization may reach components of the conduction system in their relative refractory phase, and conduction will then be slow or blocked due to a less steep phase 0.
A premature sinus node depolarization may be blocked at the sino-atrial border, and premature atrial depolarizations may be blocked in the AV node, the bundle of His or in the bundle branches.
Conducted atrial premature beats may be experienced as «a jump in the chest», but often they are not recognized. These beats are regarded as clinically innocent, and are usually not treated, but they are associated with an increased risk of future atrial fibrillation.
The AV node may respond to a premature atrial contraction in different ways: unchanged, delayed or blocked conduction. Impulses that reach the AV node in its relative refractory phase will have a prolonged PQ interval. When impulses are non-conducted, merely an isolated P wave is seen as a bump in the ECG. A premature impulse that has passed through the AV node, may normally be blocked in one of the bundle branches, resulting in a broader, aberrantly conducted QRS complex (example C below).
Since the conduction properties may differ between various parts (pathways) of the node, one pathway may be blocked while another one conducts, and this may predispose to circuit currents (reentry tachycardias) that often are induced by premature beats (see later).

For the curious reader only: Often the atrial premature depolarization front invades the sinus node and resets the timer there, postponing the next P wave. Then the next P-P interval may be slightly prolonged due to the additional time needed to enter the sinus node, reset it, and reach the adjacent atrial cells that initiate the P wave. Up to 100 ms delay may be normal. Occasionally there is a sudden, unexpected prolongation of just one PP interval. What can be the explanation?
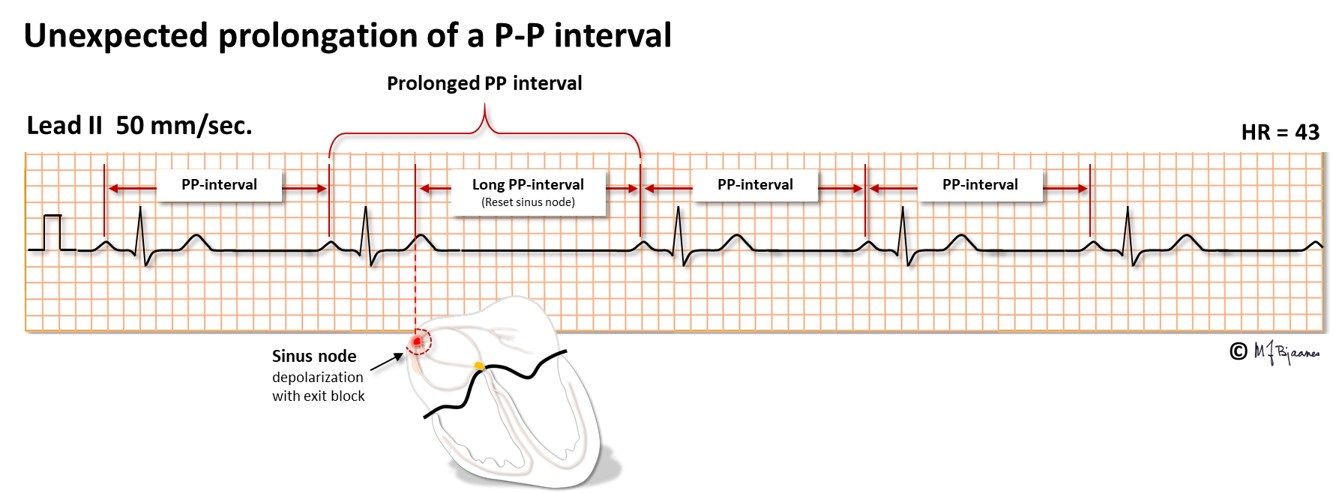
The explanation may be that the sinus node has had a premature depolarization that was non-conducted due to atrial refractoriness. The tissue mass of the sinus node is too small to give any sign of depolarization on the ECG, except for the observation that the next P wave is delayed, due to reset of the sinus node timer. Such a mechanism has been confirmed during intracardiac ECG recordings.
A heart rate >100 bpm is defined as a tachycardia, and the atria are responsible for the supraventricular forms. Usually the QRS complexes are narrow (<0.12 s), but occasionally they may be broad:
SVT episodes can often be prevented or terminated by drugs affecting pacemaker cells (calcium channel antagonists, beta-adrenergic receptor antagonists, adenosine), impulse conduction (sodium channel blockers) or the refractory periods (potassium channel blockers), and in general, the arrhythmia mechanism may be destroyed by catheter ablation. Some patients have a permanent SVT, and sustained tachycardia may induce heart failure. If you can identify the position and axis of the P waves, the mechanism of the tachycardia is usually easy to understand. An old colleague of mine used to advise us «cherchez les Ps».
Pacemaker cells are also located outside the sinus node: elsewhere in the atria, in the caval and the pulmonary veins. These may generate premature beats, and also runs of atrial tachycardia of short or long duration. As the AV node blocks impulses that are too close, it prohibits dangerously fast episodes. The P wave axis reveals the origin: lead I discerns right from left atrial origin, aVR, aVL and aVF high from low foci, and V1-V6 posterior from anterior. Regular rhythm results when P to QRS ratio is 1:1, 1:2 or 1:3, but Wenckebach conduction will result in irregular pulse.
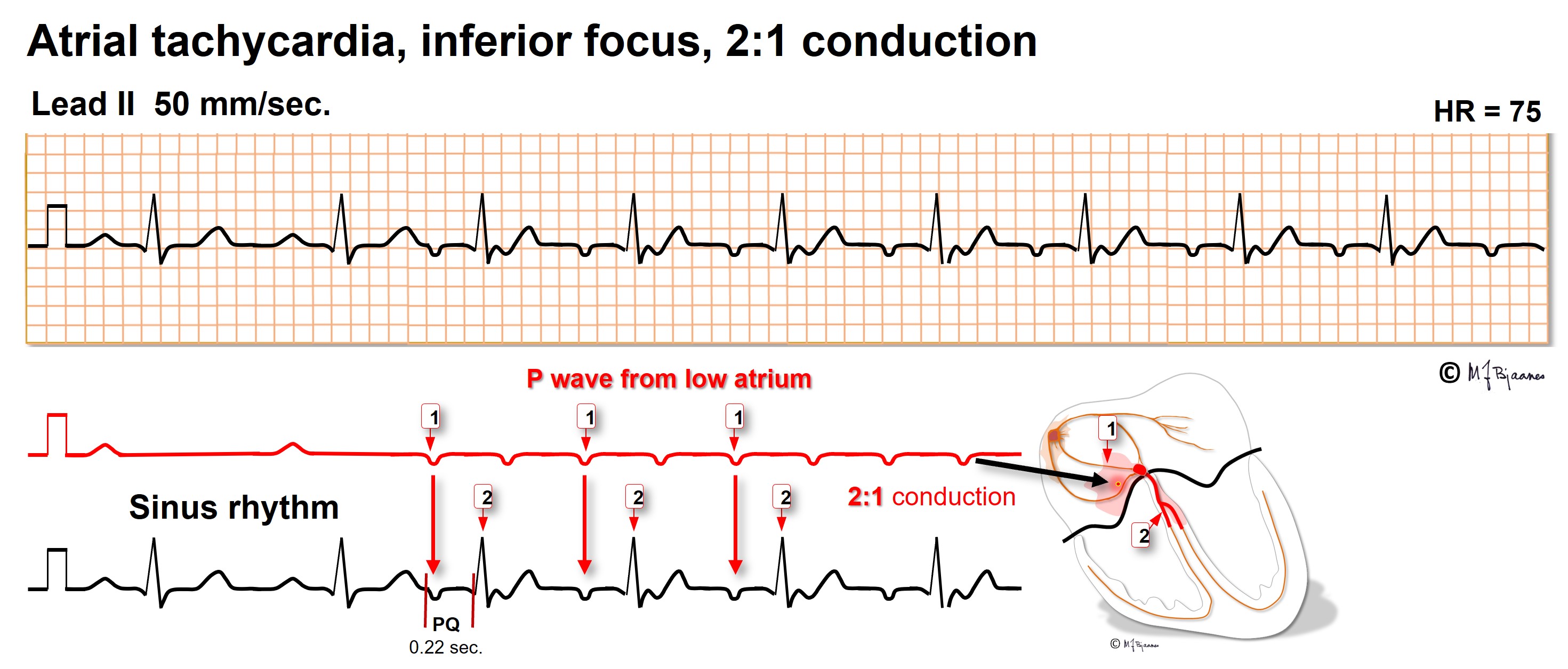
Atrial tachycardia may be left untreated when the episodes are unnoticed or well tolerated. Usually, one focus is the culprit, amenable to catheter ablation with tissue cooling or freezing. Such treatment is indicated when the tachycardia is highly symptomatic or frequent, or in persistent arrhythmia, when symptoms or signs of heart failure evolve. After heart surgery the scars predispose to reentry tachycardias (see later).
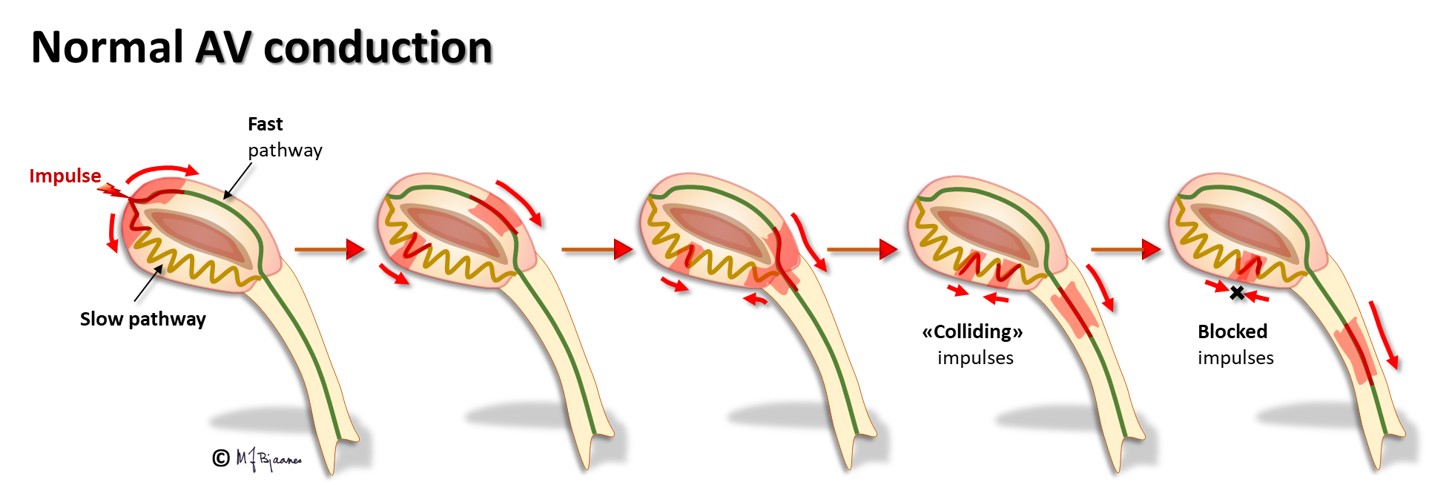
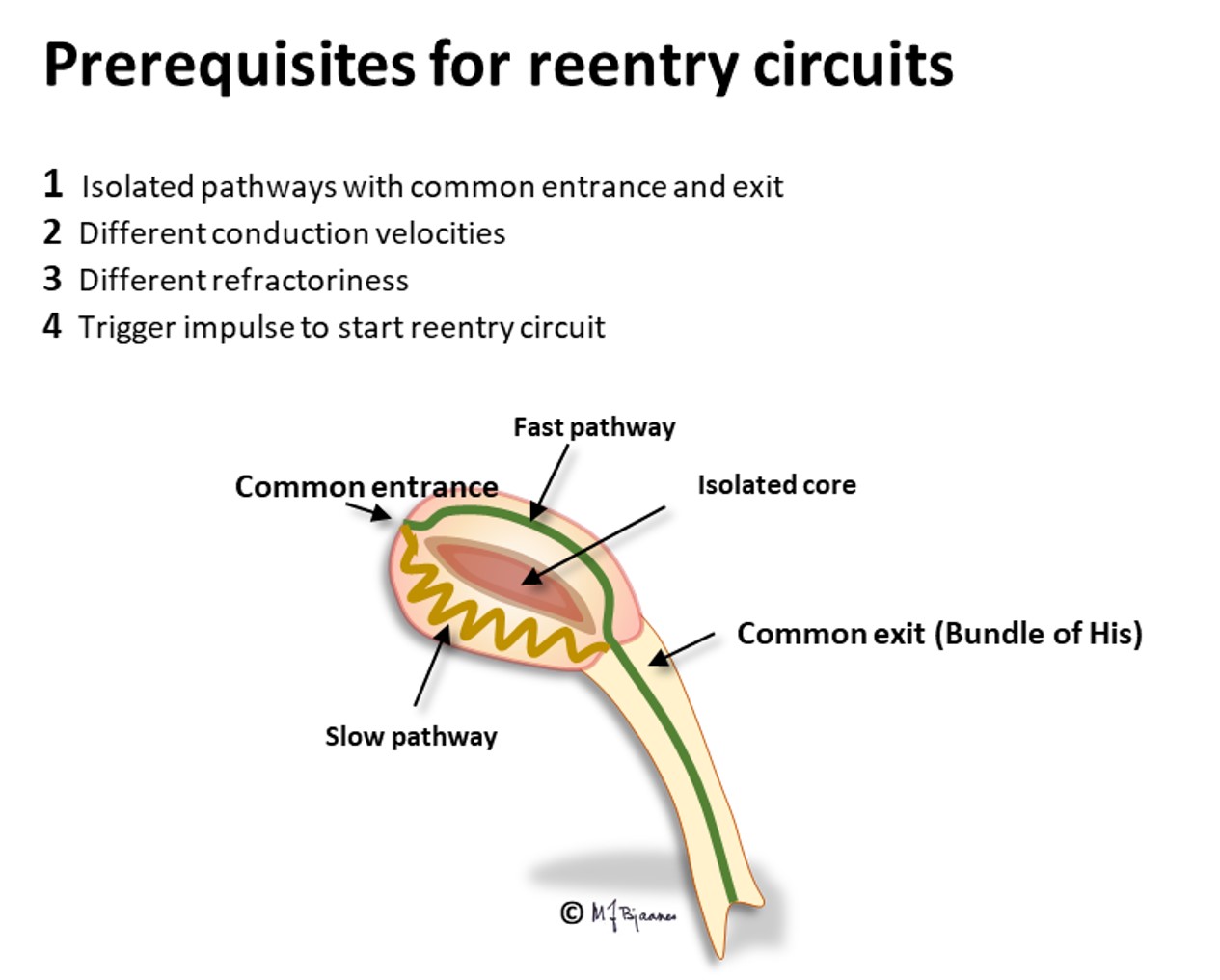
The AV node often has separated longitudinal pathways with different conduction properties. From high up anterior there is fast conduction, whereas the posterior pathway is slower. Differences in myocyte length, diameter, number of connexons and innervation explain this variation. The refractory period of the fast pathway is relatively long, whereas the slow pathway has a shorter refractory period (remember Aesop’s fable about the hare and the tortoise). An impulse from the atrium enters both pathways high up in the node, but the depolarization front that passes through the fast pathway will be the first one to reach their common exit (bundle of His). From here, it will also penetrate the slow pathway, move upward and collide with the downward propagating current; both will be extinguished.
Some persons have such pathways with highly different conduction properties. Then an early atrial premature beat may encounter a refractory fast pathway, but conduction down the slow pathway occurs. From the bottom of the node, His’ bundle is activated and a ventricular contraction follows. However, the impulse also spreads upward along the fast pathway. There is no colliding impulse here, so on the top of the node, the atria are activated retrogradely, and the now restituted slow pathway conducts downward generating the first beat of the tachycardia.
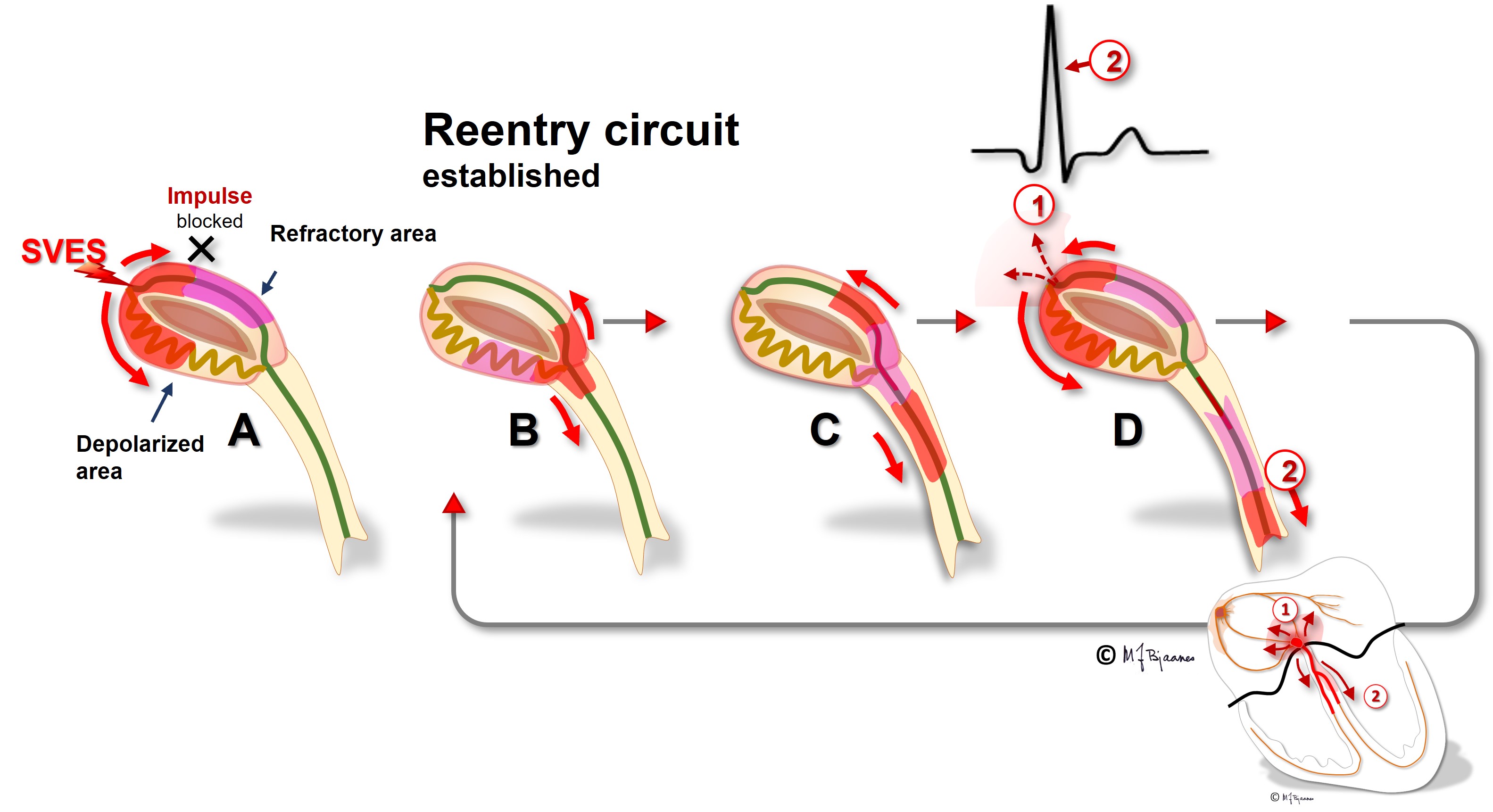
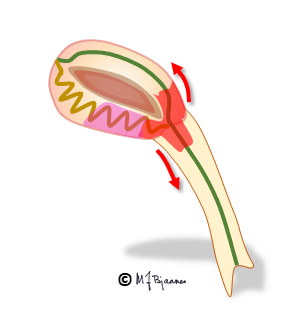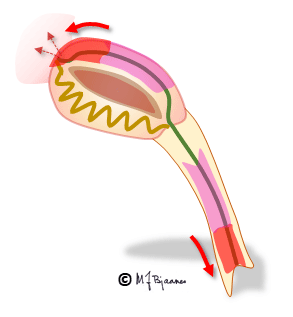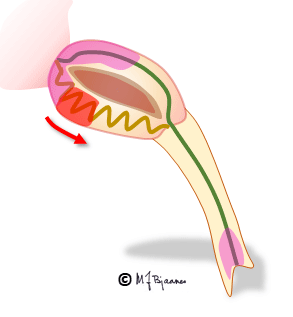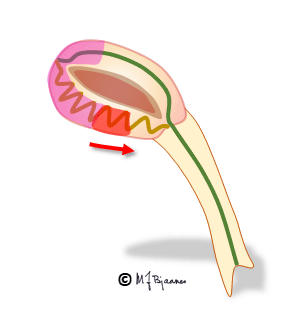
A reentry mechanism such as this one thus requires a substrate with an entrance point where impulses reach two (or more) pathways that are isolated from each other, have different conduction properties and meet at a common exit point. In addition a trigger is needed, usually a premature beat that simultaneously reaches one conducting and another refractory pathway. The tachycardia terminates by another premature beat or by changing conduction properties of the pathways, due to changes in autonomic balance or to drugs.
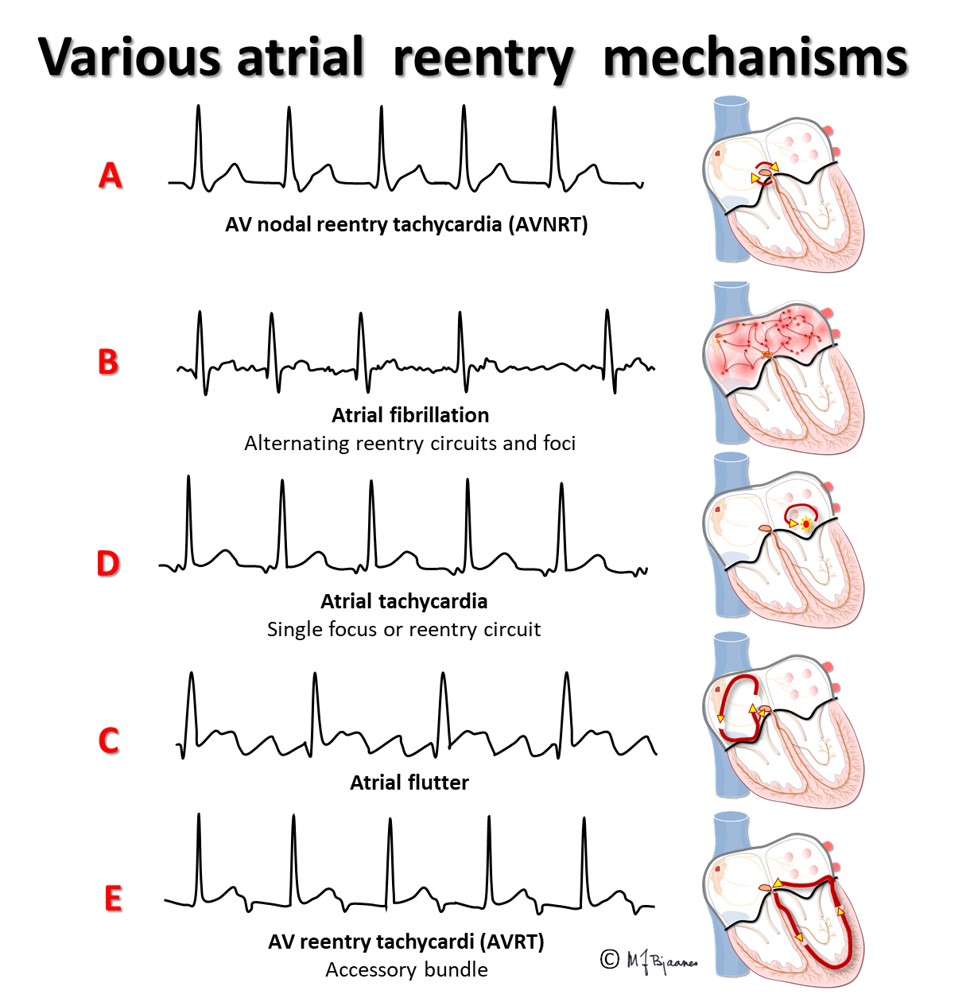
In the typical AV nodal reentry tachycardia (AVNRT) the circuit is downward the slow pathway and upward the fast one. Then the atria and ventricles are activated almost simultaneously. The P wave may be hidden within the much larger QRS complex, or part of the P wave may be seen after or just in front of the QRS.
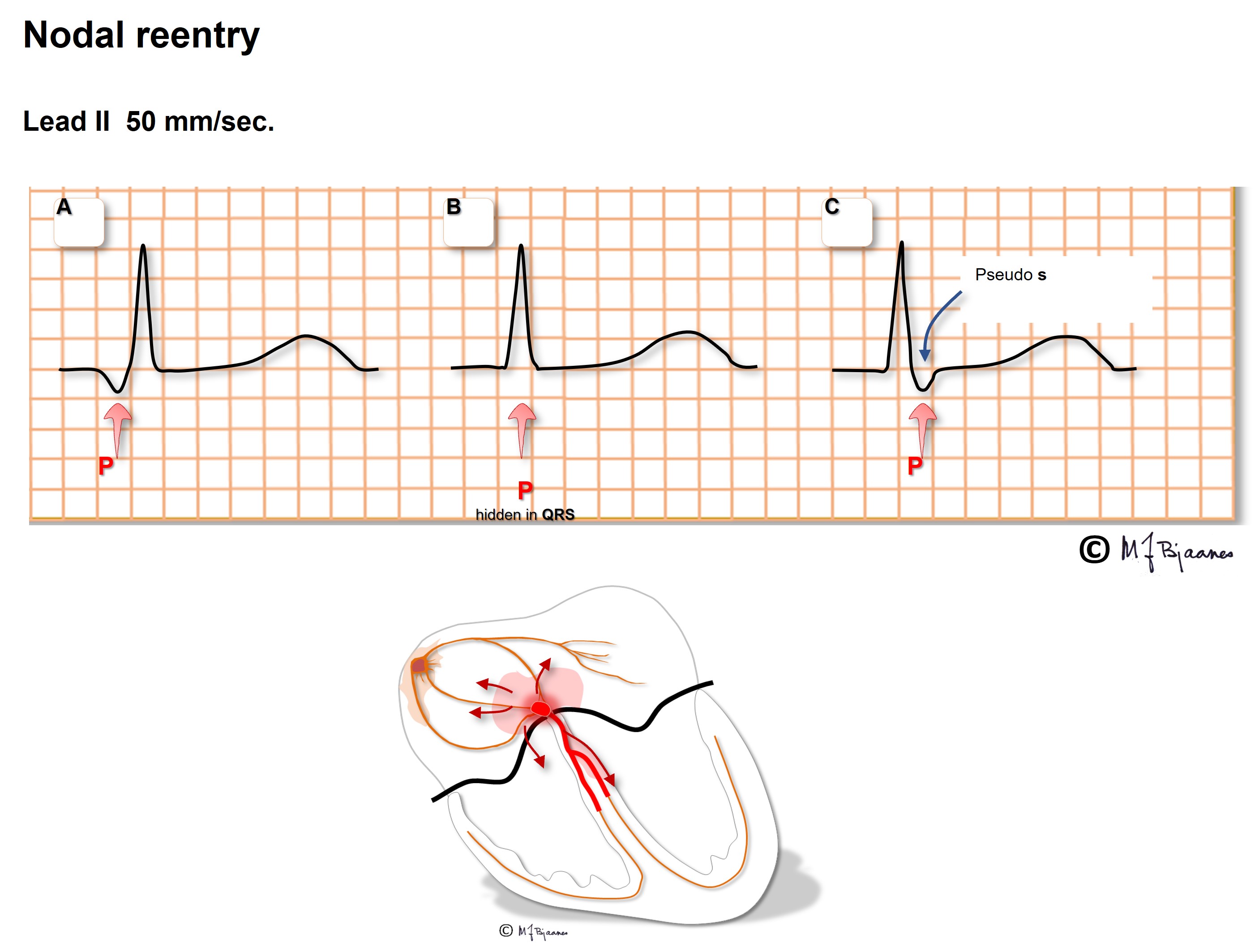
Often the P wave is easier to find when comparing the normal QRS during sinus rhythm with that during AVNRT: a tiny change in morphology may reveal the P wave.
As the atria and ventricles contract simultaneously, the pressure wave of the atrial contraction cannot penetrate the atrio-ventricular valves, but strikes backward to the caval and pulmonary veins. The patients frequently report that during AVNRT they feel pounding in the neck as well as chest discomfort, and their SVT may be erroneously diagnosed as sinus tachycardia and anxiety/hyperventilation. The tachycardia may often been terminated by Valsalva maneuver, or by a physician performing carotid artery baroreflex stimulation. Antiarrhythmic drugs are used both to prevent and terminate this tachycardia, and catheter ablation of the slow pathway usually cures this problem.
The prevalence of AVNRT in females is twice that of males, and the problem frequently occurs in otherwise heart healthy young persons, and even in children.
Patients with these arrhythmias have one or several muscle cells that penetrate the annulus fibrosus and connect the atria and ventricles. This small, thin electrical bridge (accessory pathway) comes in addition to the normal AV node – His bundle pathway. At variance from the AV node that delays the impulse, providing time for atrial emptying, the accessory bundle is a fast conductor that activates ventricular muscle cells in a small area underneath the valves prior to, and fusing with the normal QRS. The result is a short PQ (PR) interval, a broad QRS with a sloping delta wave (preexcitation) fusing with the normal QRS, and an altered T wave axis due to the abnormal sequence of depolarization.
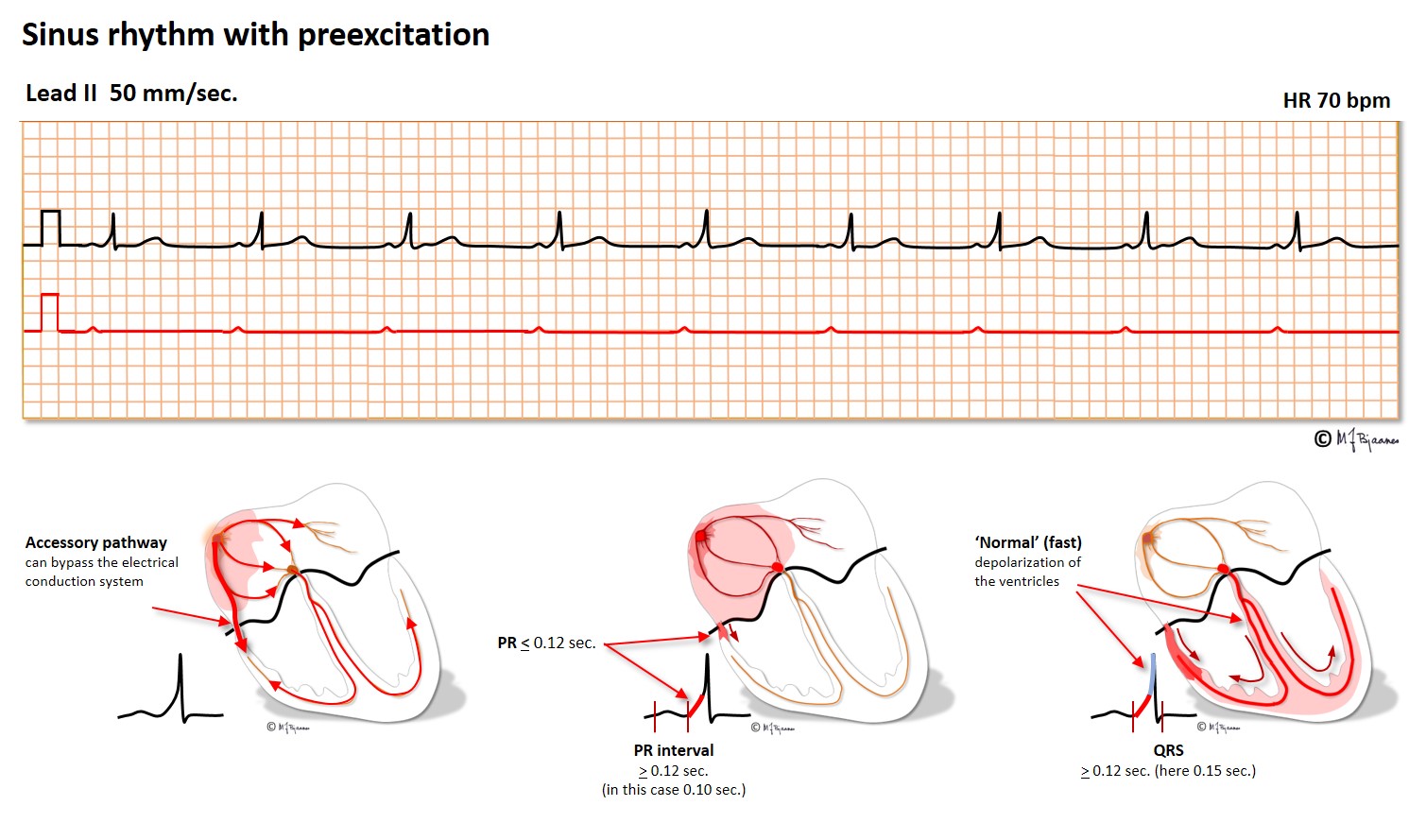
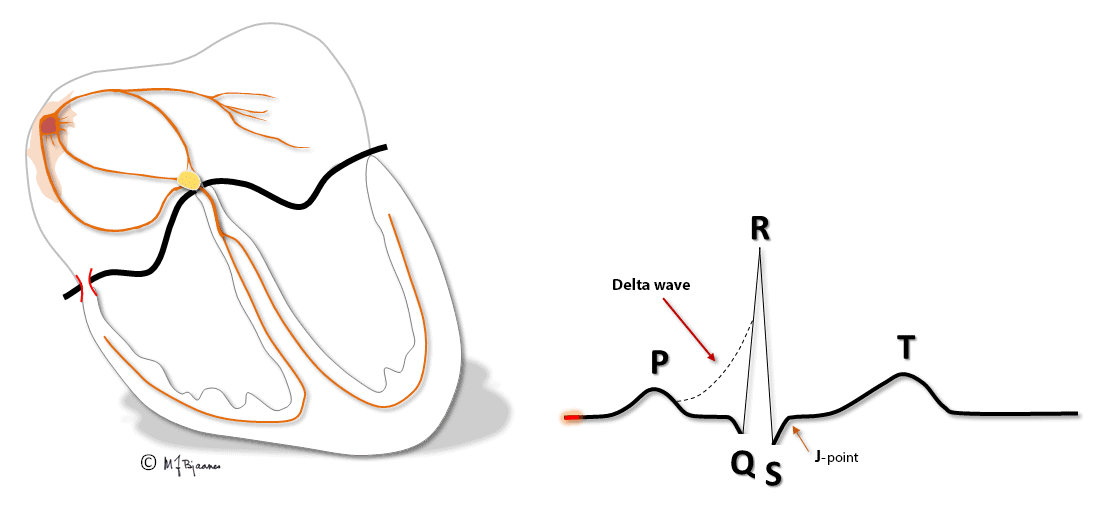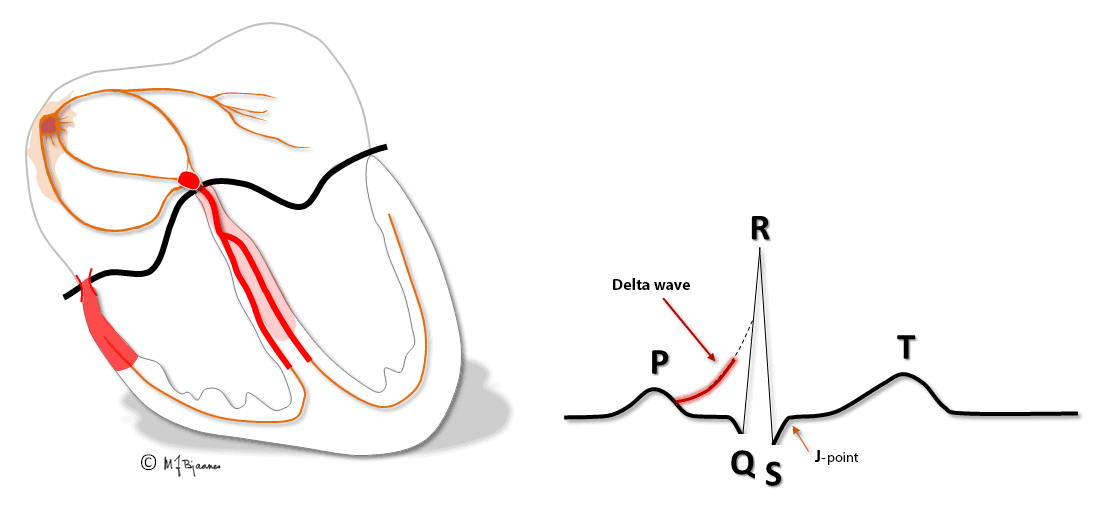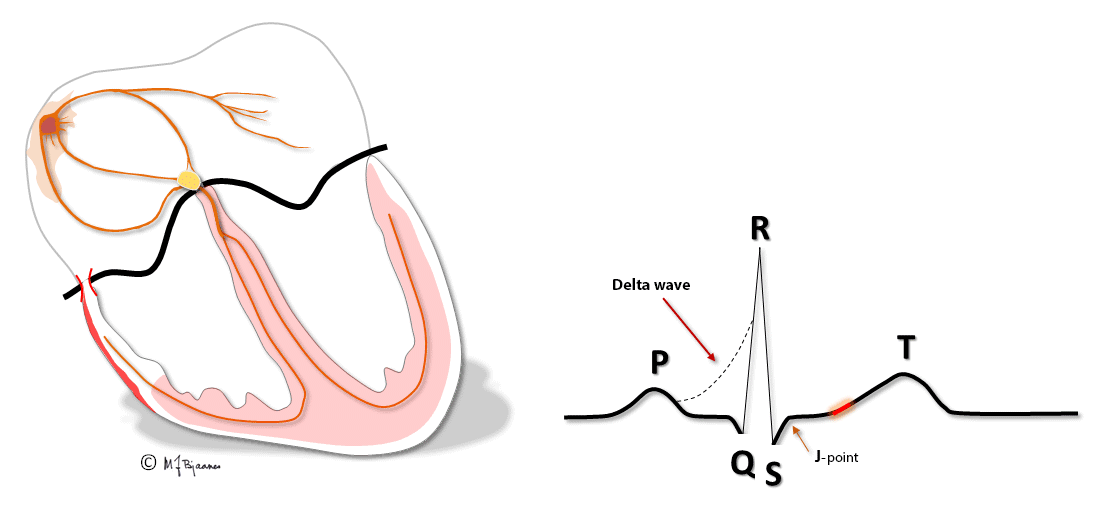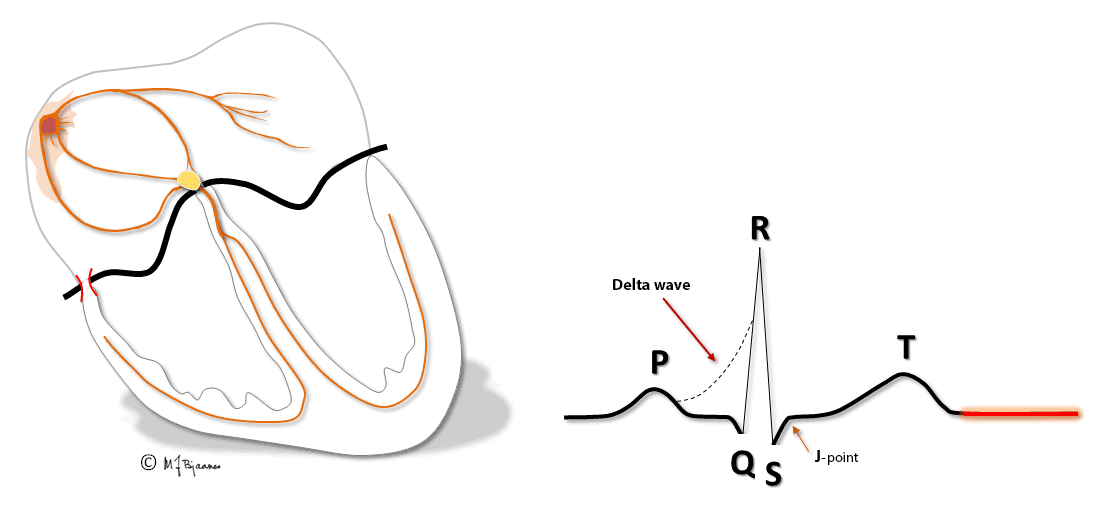
This anatomy predisposes for reentry tachycardia: there are two pathways (His’ bundle and an accessory fiber) with different conduction properties, common entrance and exit (atria and ventricles), and separated by the isolating fibrous ring. Just as in AVNRT, a premature beat from either an atrium or ventricle may reach one refractory pathway, but be conducted through the other one, and have a retrograde return allowing the rentry circuit to continue.
The common rotation is from a ventricle, up through the accessory bundle and down the normal conduction system, thus creating a normal QRS complex. This is called an orthodromic tachycardia (ortho - normal in Greek, dromo - running). Less common is a reentry circuit that spins around in the opposite direction, an antidromic tachycardia. Activation now occurs mainly outside the conduction system, and the QRS complex will look like a ventricular premature beat originating at the bundle’s insertion site.
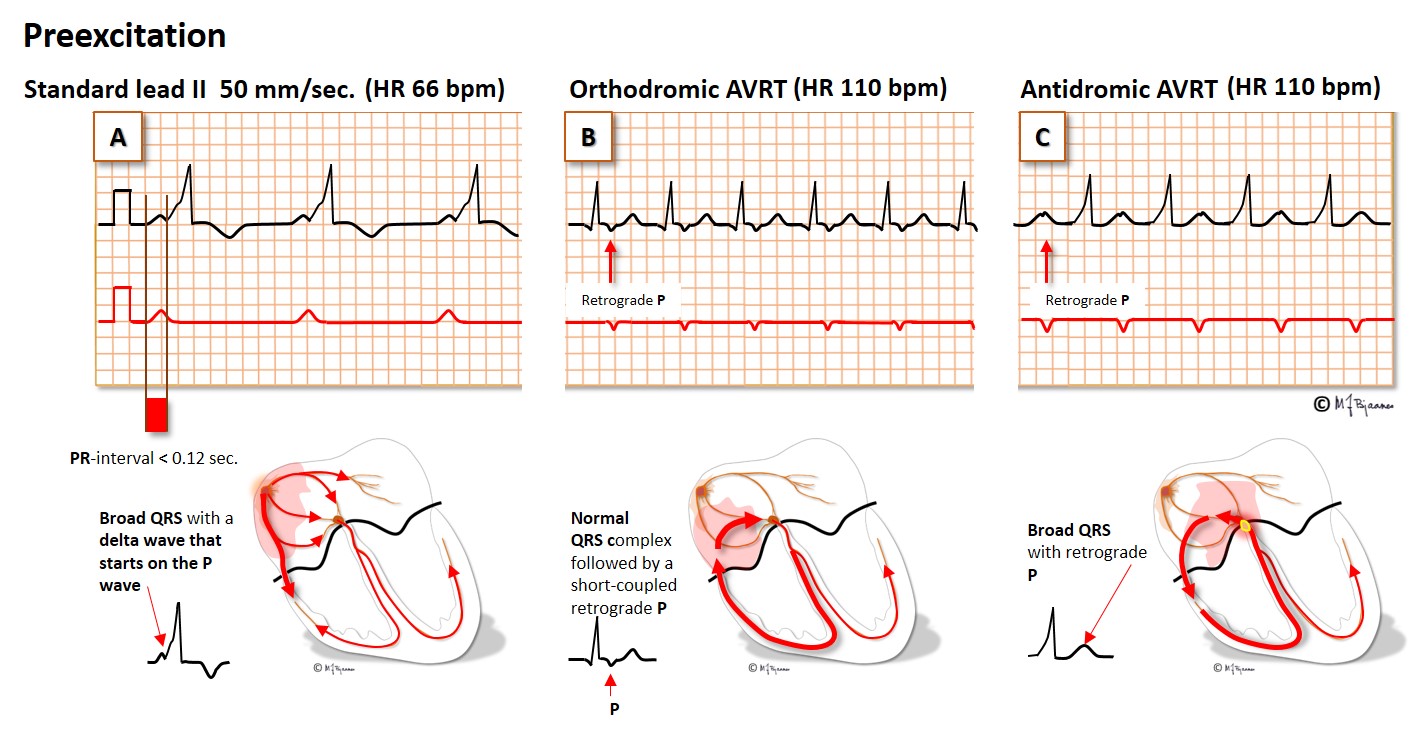
As the AV node and the atria are included in the arrhythmia circuit, there is a P wave somewhere between each QRS complex, and the tachycardia will terminate if one impulse is stopped by the AV node (Valsalva maneuver, carotid artery pressure, adenosine, verapamil or a beta blocker). When the SVT with normal QRS complexes terminates, the preexcitation returns. The correct nomenclature is atrio-ventricular reentry tachycardia (AVRT), but the combination of preexcitation and paroxysmal supraventricular tachycardia is often called the Wolff-Parkinson-White (WPW) syndrome in memory of the physicians that first described this arrhythmia.
Some accessory bundles only have retrograde conduction. Then there is no preexcitation in sinus rhythm, and never antidromic broad complex tachycardia. However, the retrograde conduction to the atria may cause orthodromic AVRT. As their resting ECG is normal, this tachycardia is called concealed WPW syndrome.
An invasive electrophysiological study permits initiation (and termination) of episodes of tachycardia, and mapping of the accessory bundle. When localized, it can be destroyed by burning or freezing (catheter ablation). Usually this procedure is uncomplicated and successful, and indicated when drug treatment is inefficient or unwanted.
Atrial fibrillation (see later) may be life threatening in a person with an accessory bundle with a short antegrade refractory period. Even asymptomatic preexcitation should therefore be evaluated by a cardiologist, and if the bundle is able to very fast conduction, prophylactic catheter ablation should be offered.
Also atrial flutter is caused by impulse reentry. The typical flutter spins around in the right atrium, upward along the septum anterior to fossa ovalis, up to the roof of the atrium, laterally around the crista terminalis and then crossing the edge (isthmus) between the inferior caval vein and the tricuspid valve, and finally, anterior to the coronary sinus ostium. Catheter ablation creates a non-conducting scar across the isthmus, breaking the arrhythmic circuit.
In typical flutter the atrial rhythm is regular, usually 250-350 bpm. The flutter waves (F waves) are generated in the right atrium, and have a characteristic sawtooth pattern in the inferior leads (II, aVF and III). The normal AV node is unable to transfer all these impulses, and fortunately block many of them. Often a regular rhythm with an A:V ratio of 4:3, 3:2 or 2:1 conduction is seen, but the intervals may also vary (Wenckebach conduction).
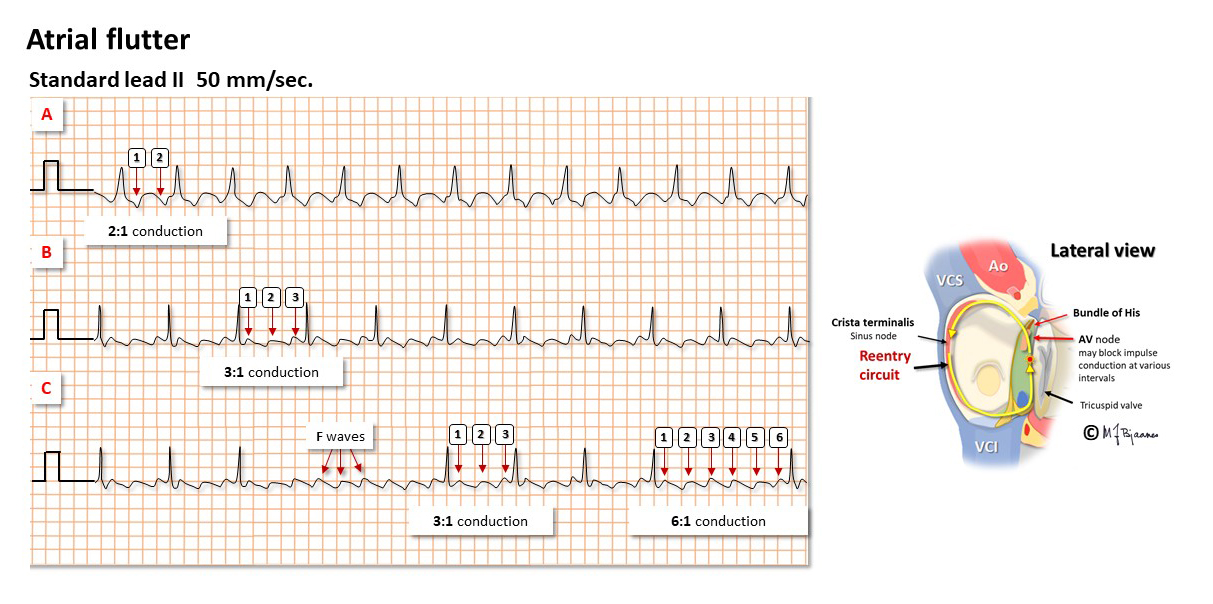
2:1 conducted atrial flutter is common. Then the ventricular rate is usually 140-150 bpm, and the flutter waves may be hard to discover. The vagus stimulation induced by carotid artery pressure may then inhibit AV nodal conduction and reveal the f-waves:

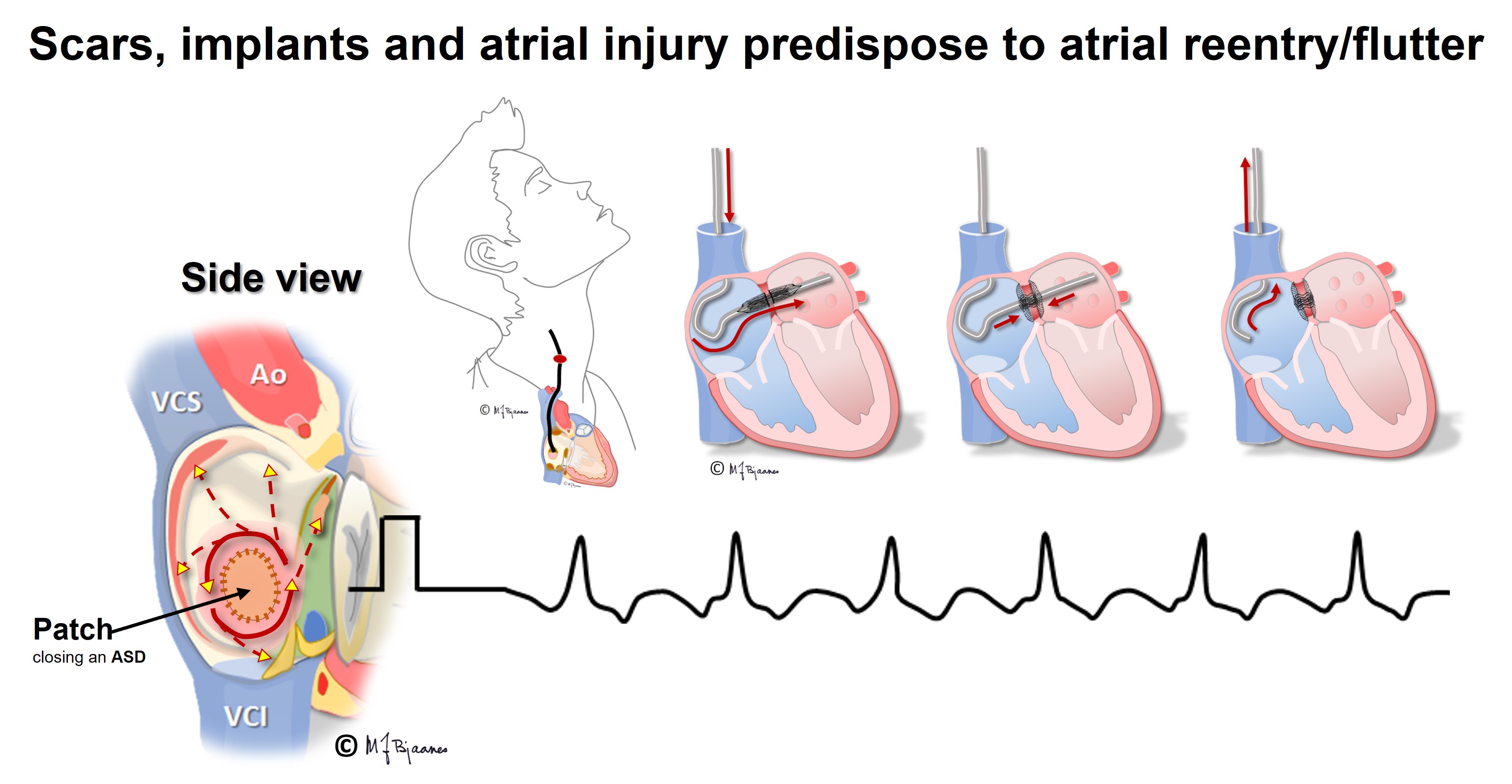
Also atypical atrial flutter is occasionally encountered: right atrial impulses may turn around clockwise, or around atrial scars created by heart surgery or extensive catheter ablation in the left atrium (to cure atrial fibrillation).
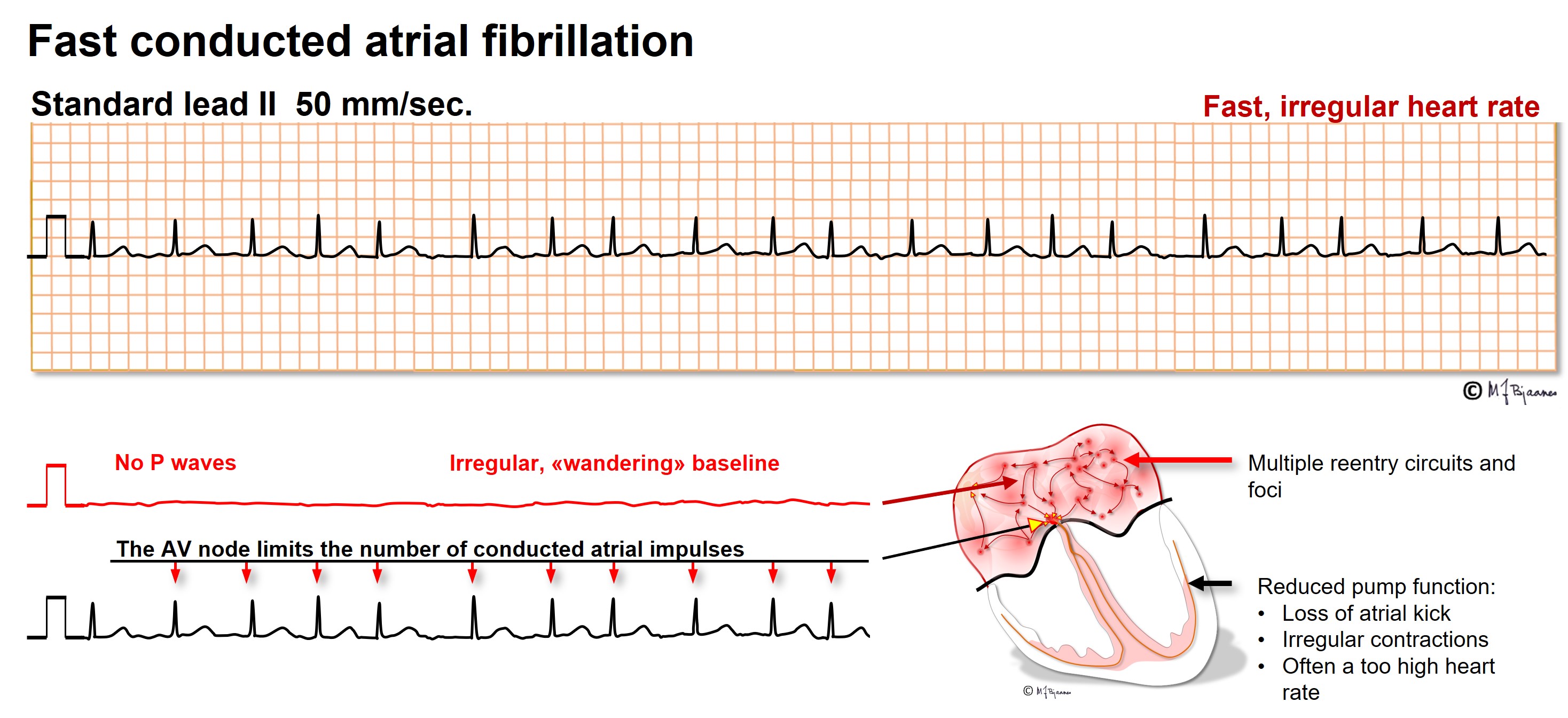
AF is characterized by fast, irregular (chaotic) atrial contractions (>350/min) and irregular QRS complexes. ECG is the diagnostic tool, and an irregular pulse should raise suspicion of AF. In recent onset AF the fibrillatory waves (f waves) may be marked and look like P waves in some leads (usually V1-V2). In longstanding AF the baseline is almost straight.
Arrhythmologists often regard atrial fibrillation as «the mother of all arrhythmias», since all arrhythmogenic factors may be involved: there is a substrate (reentry is facilitated by dilated fibrotic atria), the triggers are there (premature atrial beats, either from pacemaker cells or from triggered automatism in ordinary myocytes), and finally, autonomic or local factors (vagal and sympathetic activity, ischemia, electrolyte disturbances, hypovolemia). The ventricular rate is usually fast in recent onset AF, but often normalizes with time. Frequently patients need rate reducing drugs. These delay or block AV nodal conduction (beta blockers, calcium channel inhibitors). To prevent onset of AF, drugs must reduce impulse conduction (Na+ channel blockers) or increase refractory periods (K+ channel inhibitors). During catheter ablation of AF, left atrial (scar) lines of block are created around the pulmonary veins’ inlet to prevent AF induction by premature beats from pulmonary venous sites.
When a person with a previous bundle branch block has AF, the tachycardia will, of course, also have broad QRS complexes, and may be erroneously regarded as a ventricular tachycardia (VT). The AF is, however, characterized by a typical bundle branch block morphology (either right or left), and the R-R intervals vary much more than in VT. Occasionally a broad QRS complex is seen in AF, within a series of narrow complexes. This usually happens when a long R-R interval is followed by a short one. The latter is aberrant because it hits the relative refractory period of the previous beat, that is prolonged due to the preceding long interval.

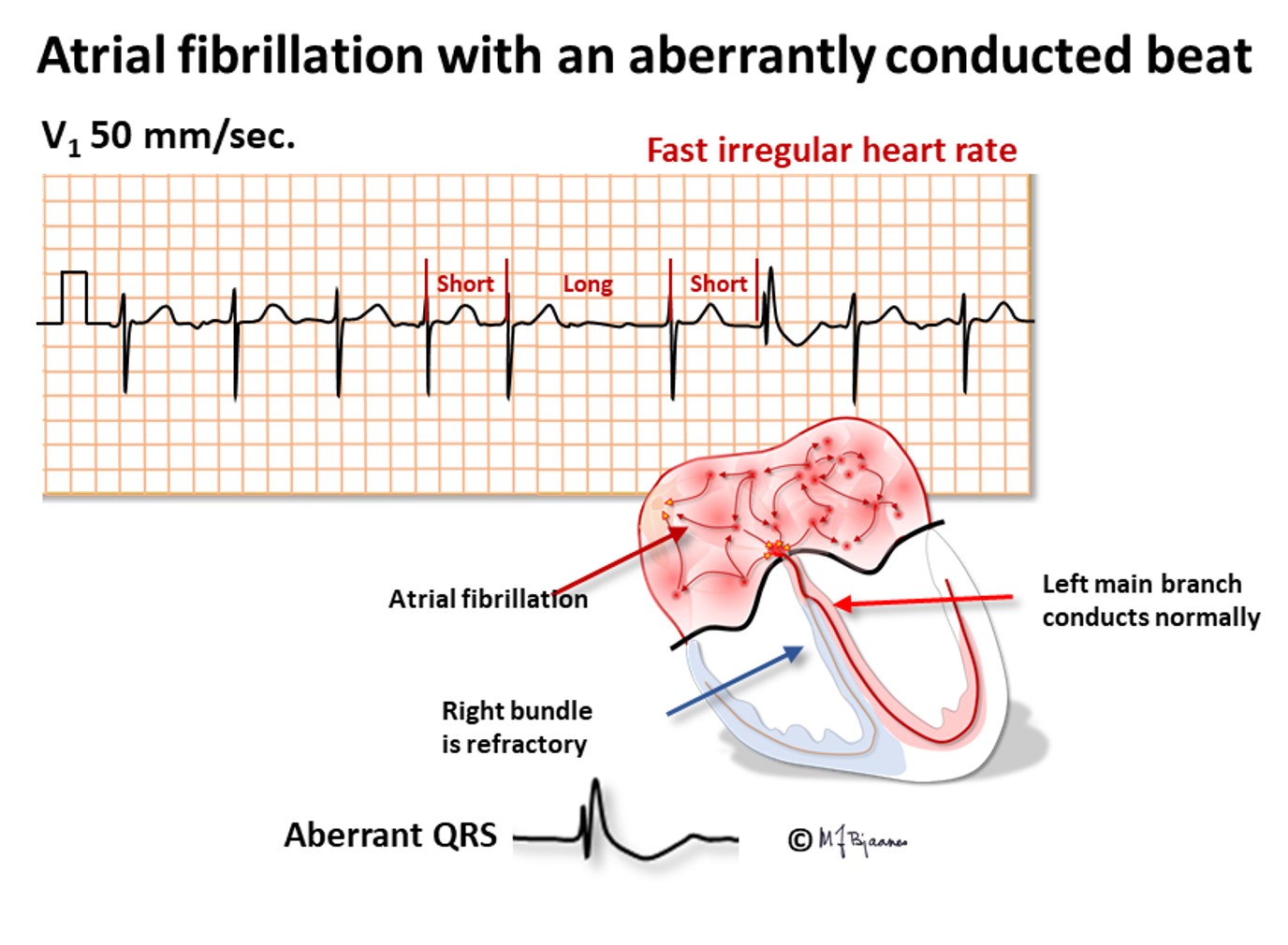
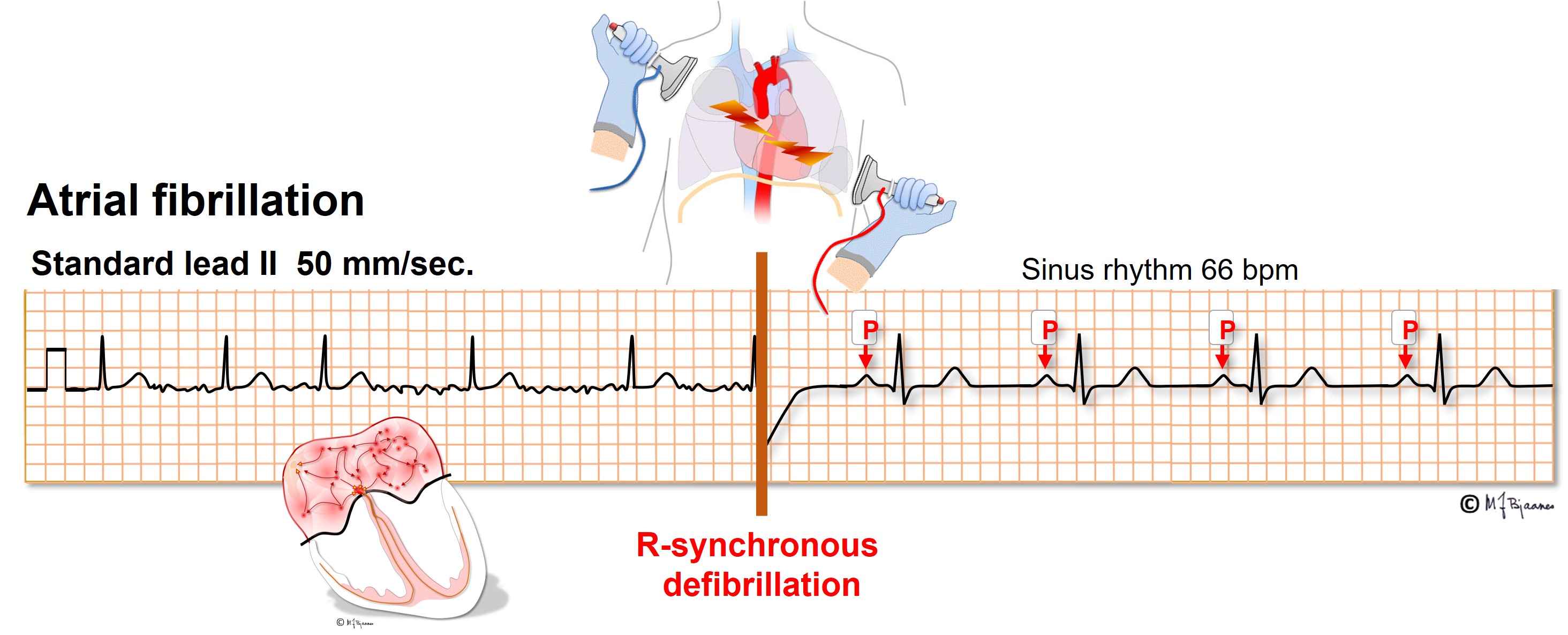
Atrial fibrillation occurs before or later in 1/3 of all aged 40+. Heart disease and hypertension predispose, as do intensive physical training and alcohol intake. AF may be paroxysmal, sustained or permanent. It is important to recognize AF because elderly and heart sick patients with AF suffer from increased risk of stroke and dementia, preventable by anticoagulant drugs. Recent onset AF may often be terminated by DC current. Administration of an R-synchronous shock is important to avoid an R-on-T shock that may induce ventricular fibrillation (see later).
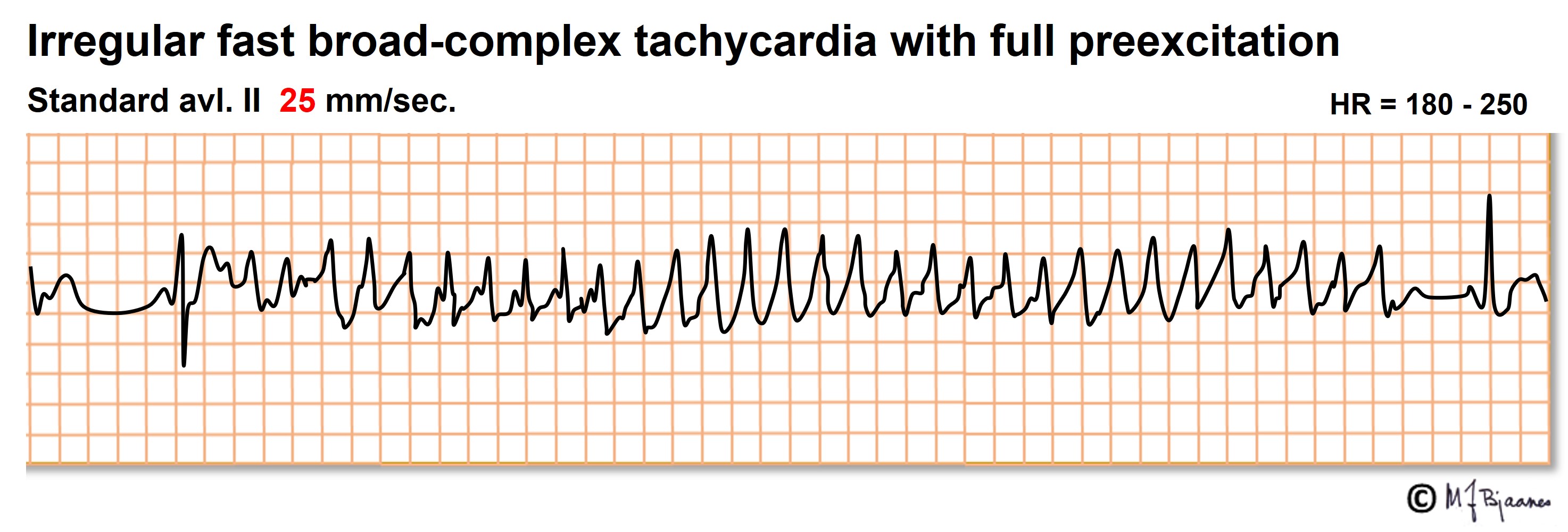
An accessory bundle may conduct AF impulses extremely fast, resulting in a life threatening irregular broad complex tachycardia. Emergency DC cardioversion is then needed.
VPBs are heart beats that are non-conducted, i.e. generated within the ventricles. They can arise from small cell groups (automatism) or by myocardial reentry. Sometimes ventricular impulses penetrate the conduction system and give a retrograde P wave, often resetting the sinus node. When atria and ventriclecontract simultaneously, the retrograde pulse wave may be felt, just like in AVNRT. VPBs that occur at rest and disappear during exercise are regarded as innocent, but if they first appear during exercise, they may herald a heart disorder. A great number VPBs (for instance >>6000/24t) may in the long run disturb cardiac work. One VPB in a ten second recording corresponds to 1 by 6 by 60 by 24 (8640) VPBs/24 h.
VPBs usually lack a preceding P wave, and differ from typical right as well as left bundle branch block. They are broad (≥0.12 s, usually >0.14 s), as they propagate outside the conduction system. The initial slope (R or S) is gentle because they start from a focus, but as they often penetrate the conduction system, the terminal slope is steep. The time from q (or R) onset to R peak is longer than the interval between S bottom and the J point. In a QRS with bundle branch block the opposite is the case.
VPBs may occur at random, but often they have a fixed interval to the previous beat; they are coupled. When the latter pattern is regular, it is called bigeminy, and a sequence of three is trigeminy.
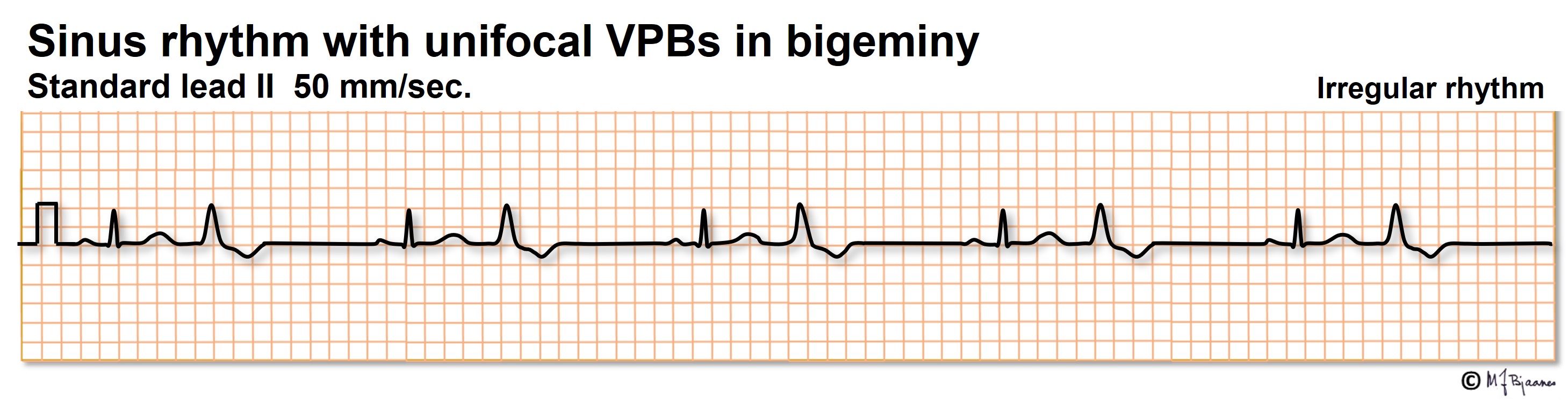
A VPB may have retrograde conduction to the atria, and even enter and reset the sinus node. This may result in short (normal) and prolonged P-P intervals:
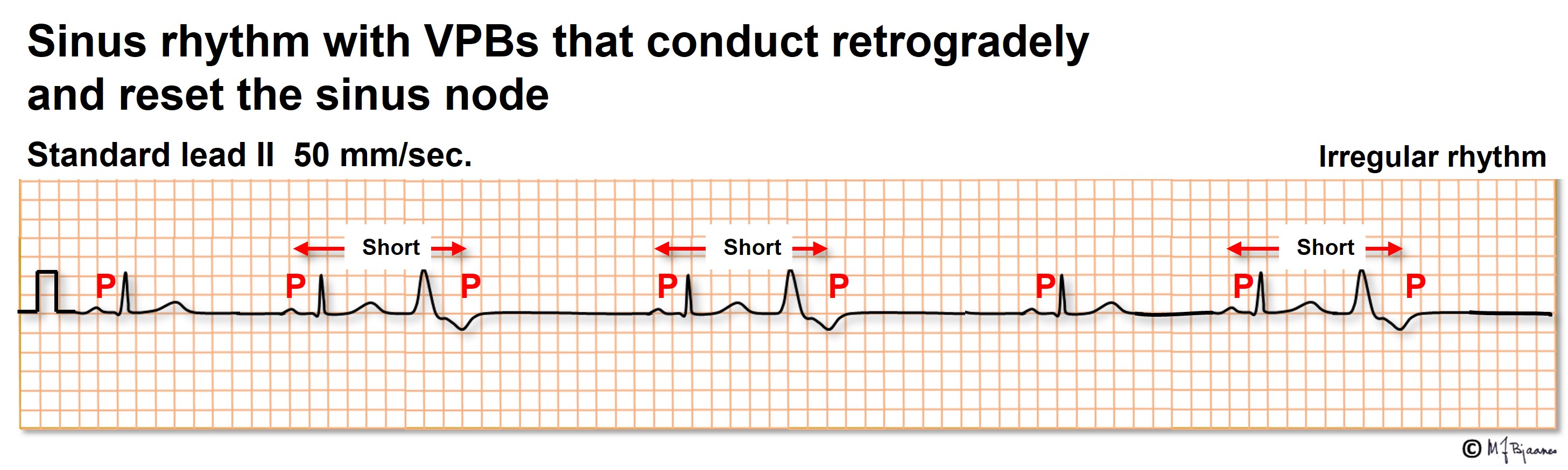
A VPB without retrograde conduction allows the normal sinus beats to continue without reset. Even a VPB that is interposed between two regular sinus beats need not disturb the rhythm further on:
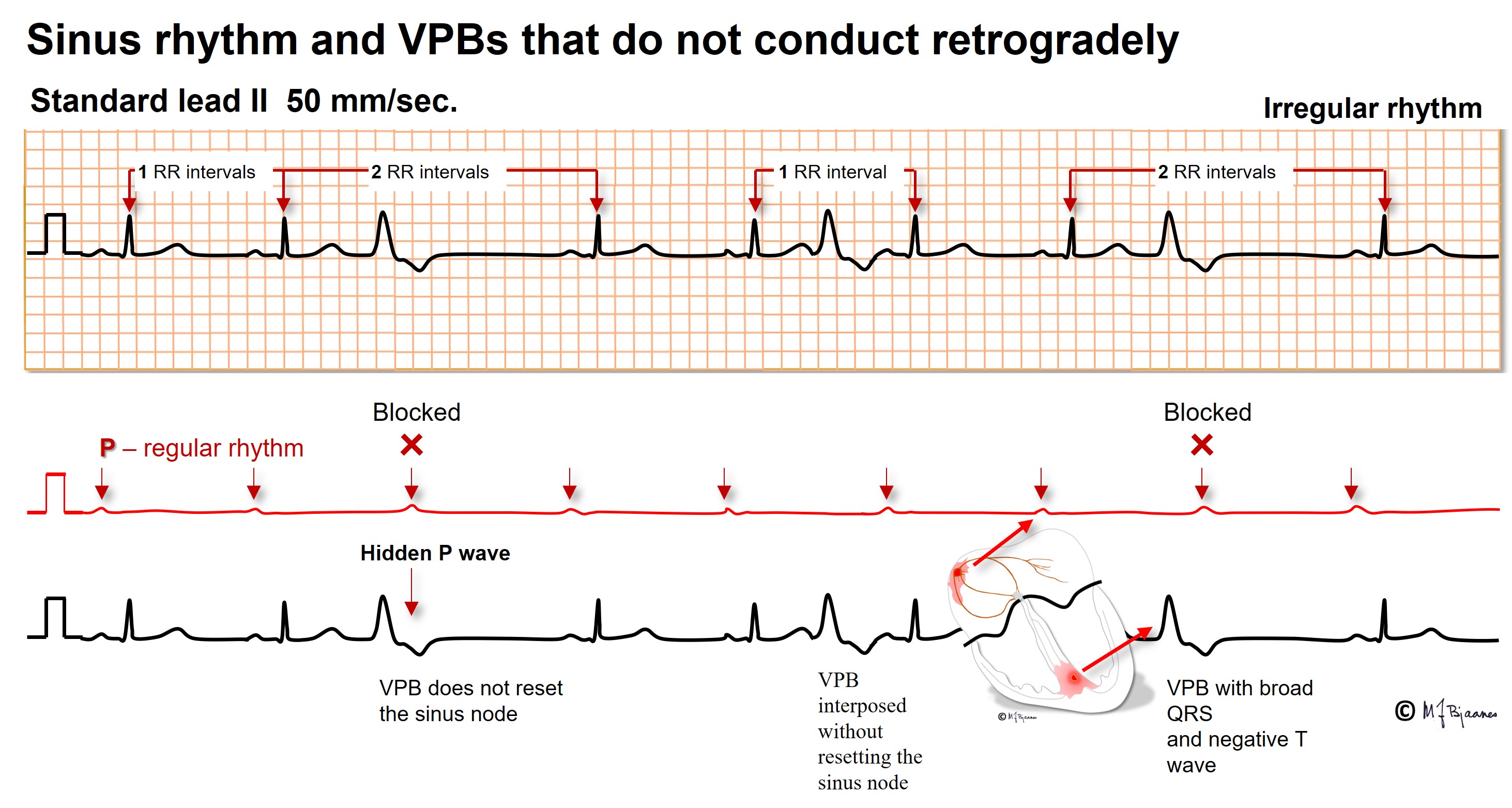
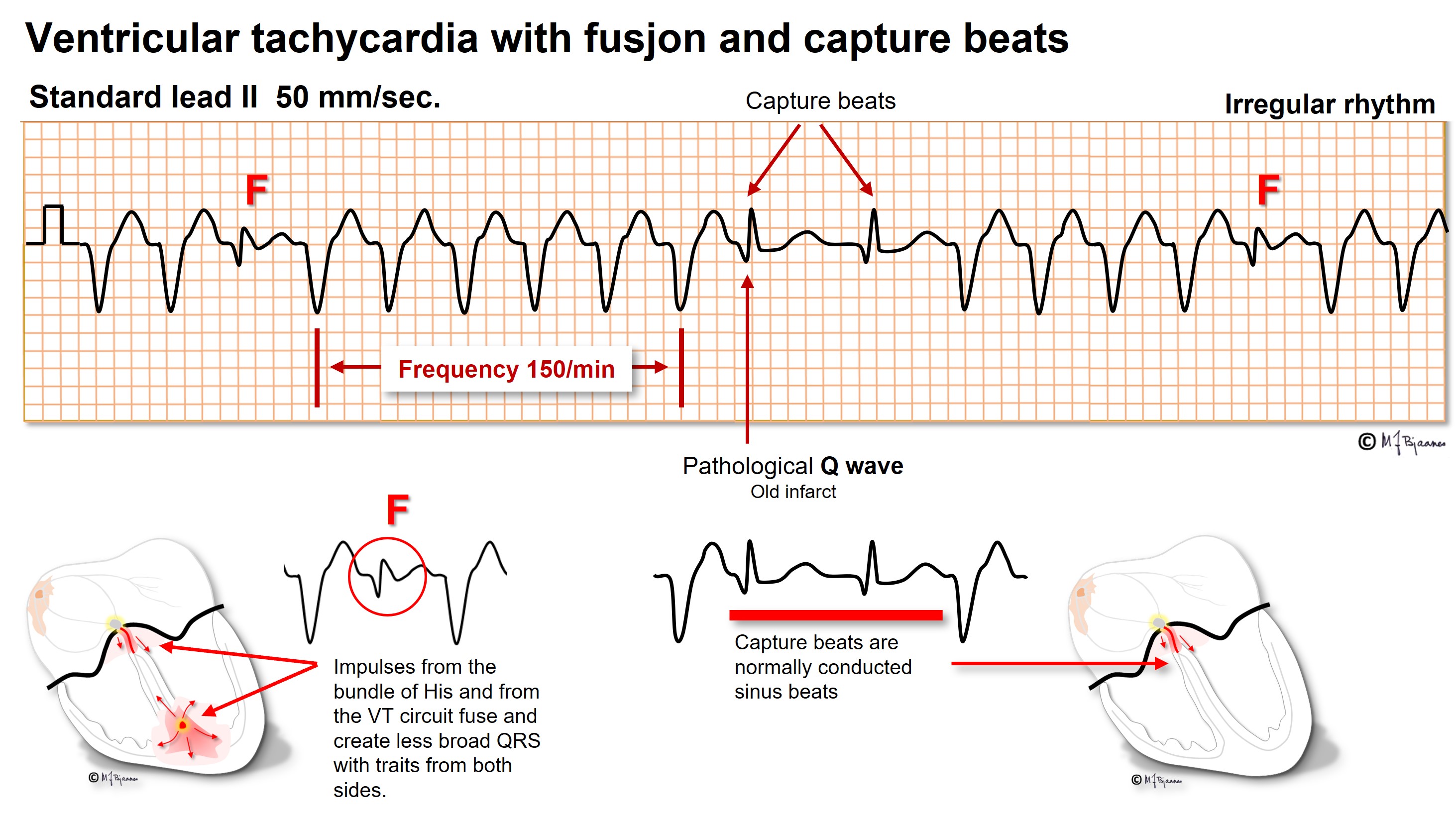
A VPB may further activate the ventricle simultaneous with a conducted impulse, and then the resulting QRS is a mixture of the two, a fusion beat. Note that below, the sinus rhythm is unaffected, but the P waves are hidden within the T waves of the VPBs and are non-conducted due to a refractory conduction system. The F complexes are fusion beats: they are not as broad as the VPBs due to the supraventricular contribution, but the inverted T wave of a VPB confirms the fusion.

VPBs usually have a similar morphology, they are monomorphic, but if they arise from several foci or reentry circuits, they are polymorphic and herald a worse prognosis.
When >3 ventricular beats occur in a series, the patient has ventricular tachycardia (VT). A run of up to 15 beats is non-sustained VT, and >15, sustained VT. A few ventricular tachycardias may occur in otherwise healthy hearts, and carry an excellent prognosis, but the majority of VTs express an underlying heart disease and reflect a high risk of sudden death (ventricular fibrillation). While supraventricular tachycardia (SVT) usually can be treated with verapamil, beta blocker or adenosine, these drugs may induce hypotension in VT, and in sick patients, anesthesia and DC cardioversion is often the safer treatment. VT prevention by drugs is rarely successful. In a few cases catheter ablation of the reentry circuit is possible, but usually an implanted cardioverter/defibrillator (ICD) should be considered.
Therefore a correct diagnosis of a broad complex tachycardia is important. In VT the QRS complexes are almost always ≥0.12 s (and usually ≥0.15 s) and fairly regular. Often atria and ventricles contract independently. In a broad complex tachycardia VT is almost always the diagnosis when
VT is the probable diagnosis when
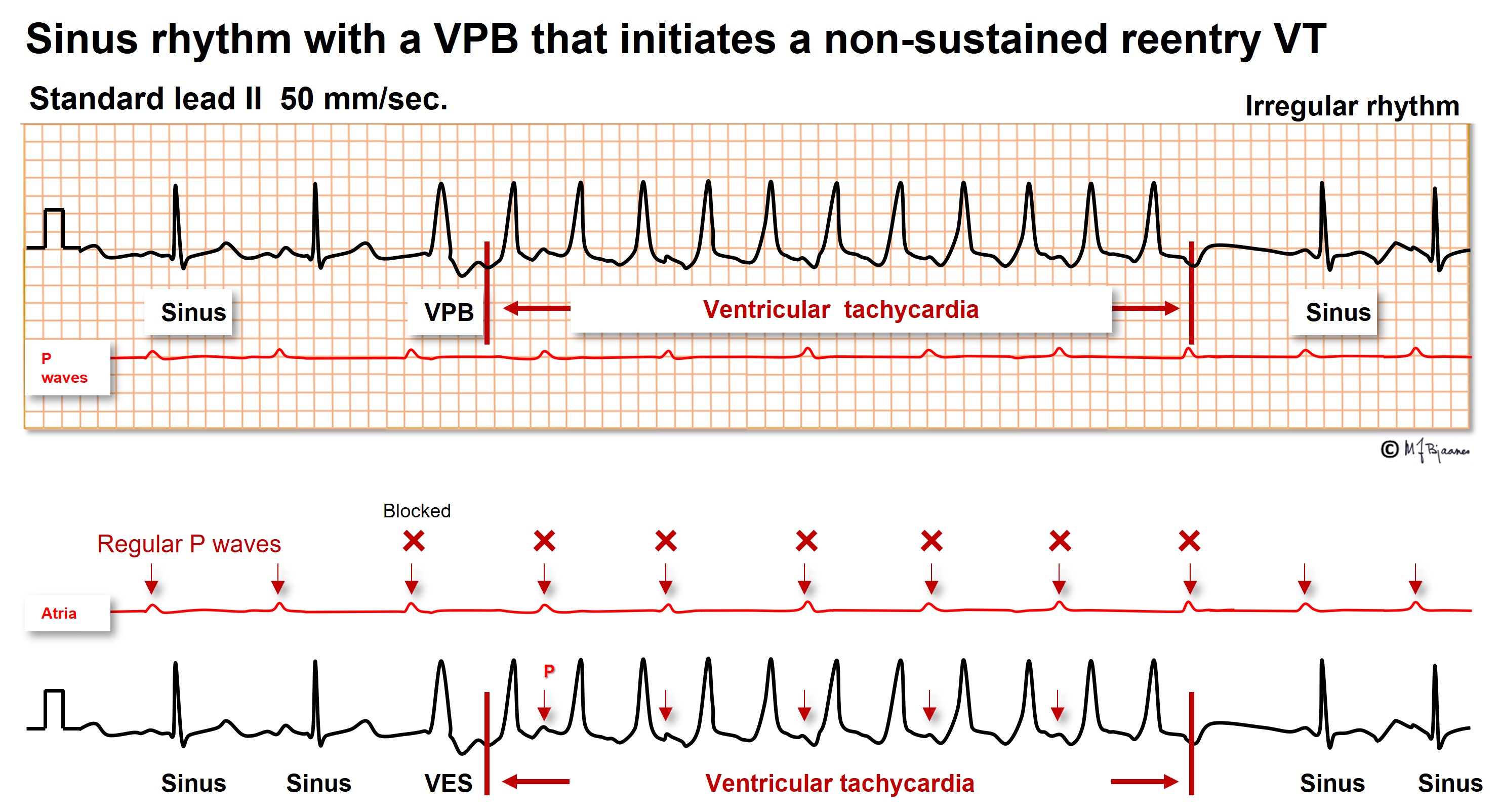
Here there are two sinus beats, then a VPB that initiates the reentry VT. Between every other QRS a retrograde P wave is easily seen. When there are more QRS complexes than P waves, a tachycardia is certainly a VT.
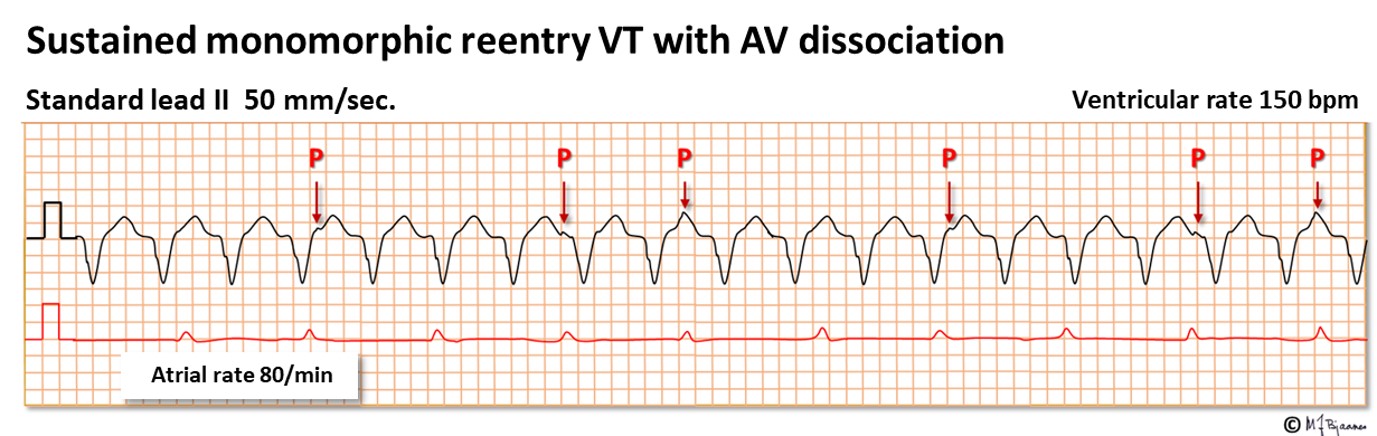
The illustrations above both show a regular broad complex tachycardia. In the upper one, a non-sustained VT, P waves are easily seen, but in the lower, merely an occasional tiny bump represents a P wave. Some P waves are buried within the QRS complexes or T waves. When seen, the intervals are usually multiples of the shortest PP interval. Here the atria are stimulated by the sinus node, whereas the ventricles run their independent rhythm. The AV dissociation proves the VT diagnosis. Often a long rhythm strip is required to find the P waves, and the best is often to record a few sheets of 12 channel ECG for scrutiny.
Good algorithms are available to discriminate between VT and aberrantly conducted SVT (aberrant means conduction with bundle branch block due to the tachycardia). Precise diagnosis requires, however, specialist competence (Vereckei A et al, New algorithm using only lead aVR for differential diagnosis of wide QRS complex tachycardia. Heart Rhythm 2008;5:89 –98). In practice, it is safest for the less experienced to treat all tachycardias with QRS >0.12 s as if they were a VT, that is, with anesthesia and R-synchronous C cardioversion.
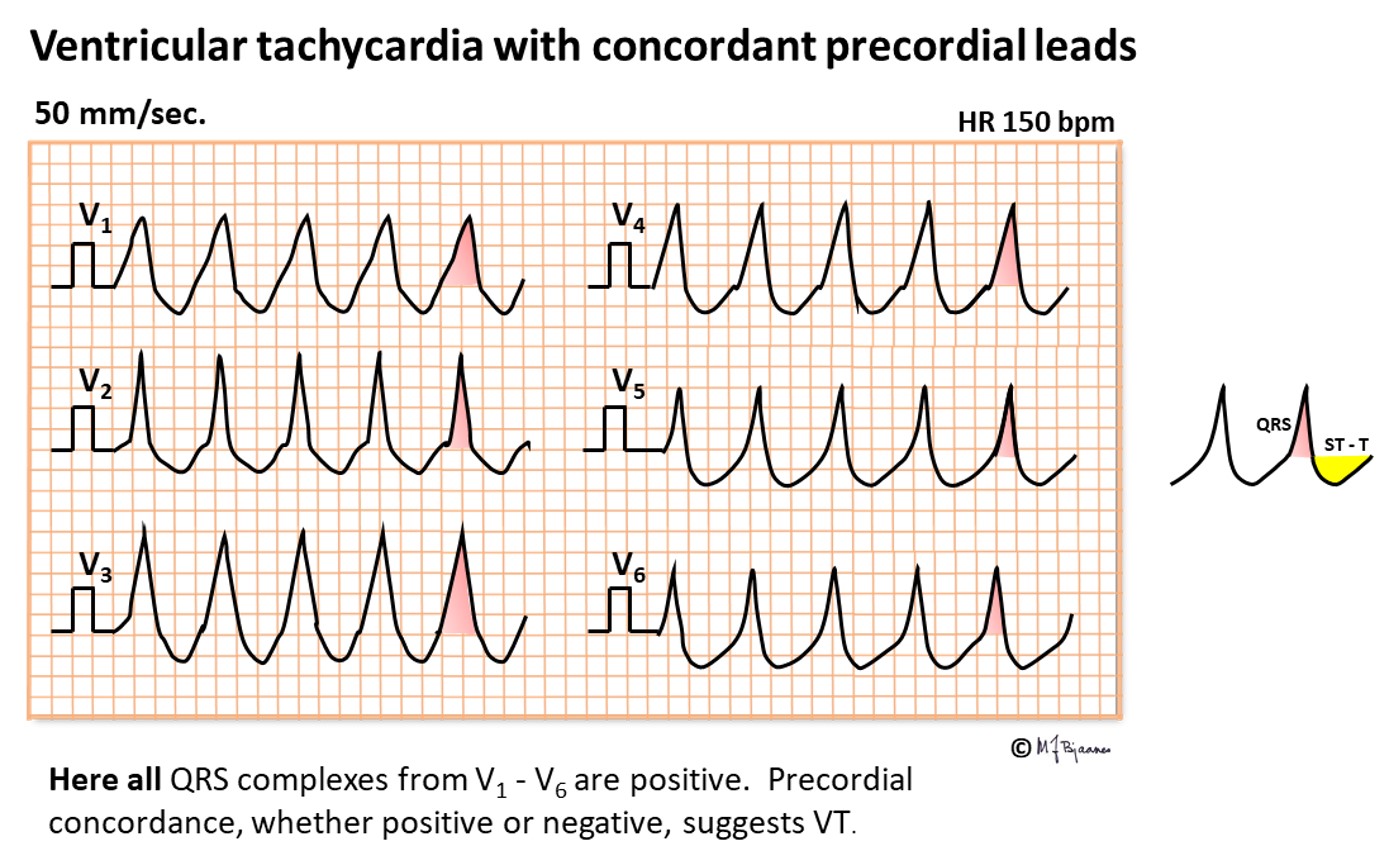
When QRS complexes are bizarre and markedly differ from bundle branch patterns, VT should be strongly suspected. For instance, when all precordial leads are concordant (all positive or all negative), their activation cannot have been transmitted by the conduction system.
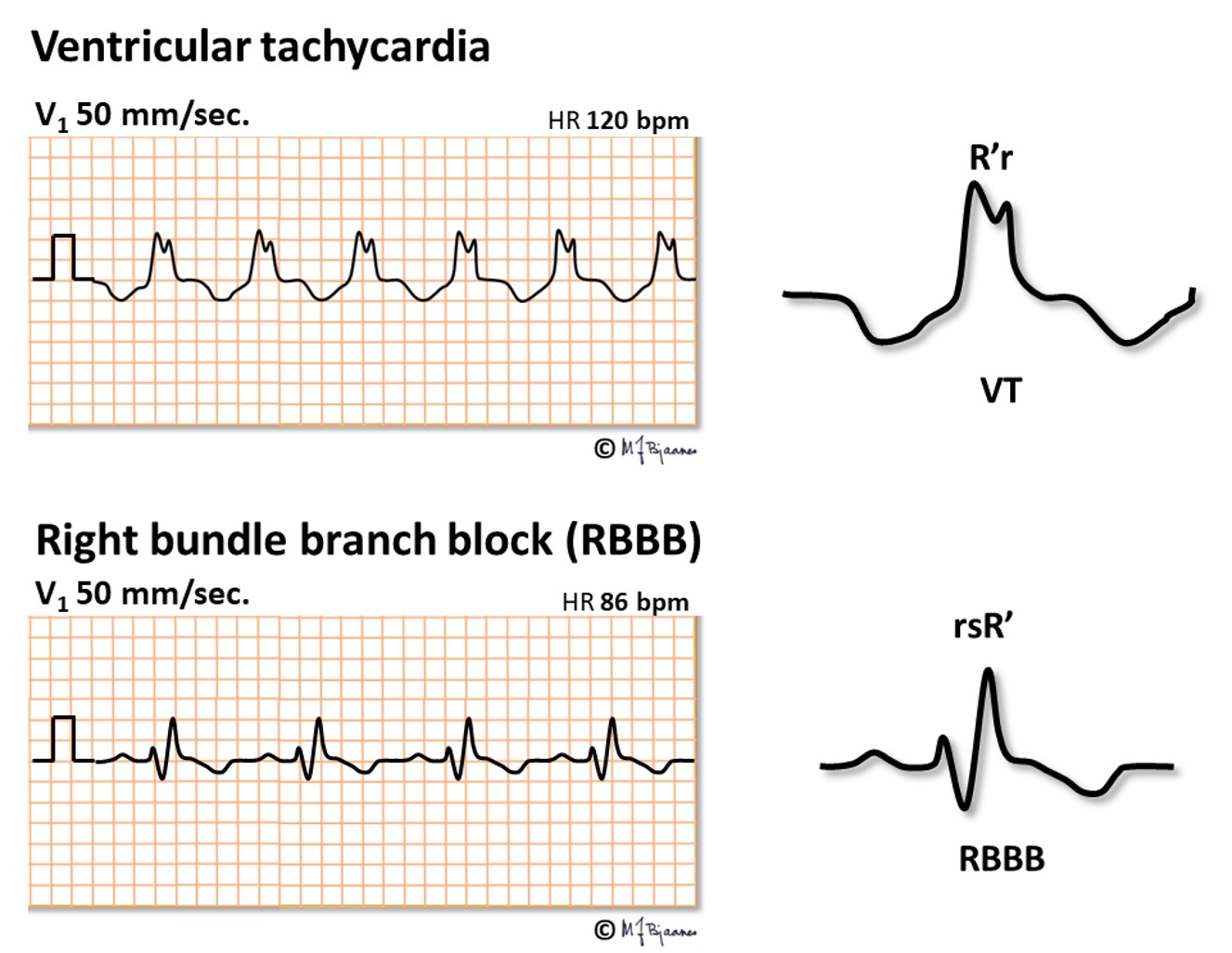
When broad QRS complexes look like bundle branch blocked QRS, the may represent an aberrantly conducted SVT even if no P waves are seen; these may be hidden within the QRS complexes or, at fast heart rates, in the preceding T wave. Lack of similarity with BBB, however, suggests VT. Below, the first peak in V1 (R) is higher than the next one (r’), and that is not the case in bundle branch block.
Some ventricular tachycardias are short rapid bursts of broad QRS complexes that may cause dizziness or syncope, and if long-lasting, they may trigger ventricular fibrillation (heart arrest). «Polymorphic» means multiple QRS morphologies, usually a gradual change of axis, as if twisting around the baseline (torsades de pointes VT). The mechanism of this arrhythmia is usually «triggered automatism», as the action potentials have got an afterdepolarization that can trigger a premature beat (VPB), which may trigger the next beat, and in combination with reentry mechanisms, the VT goes on until extinction.
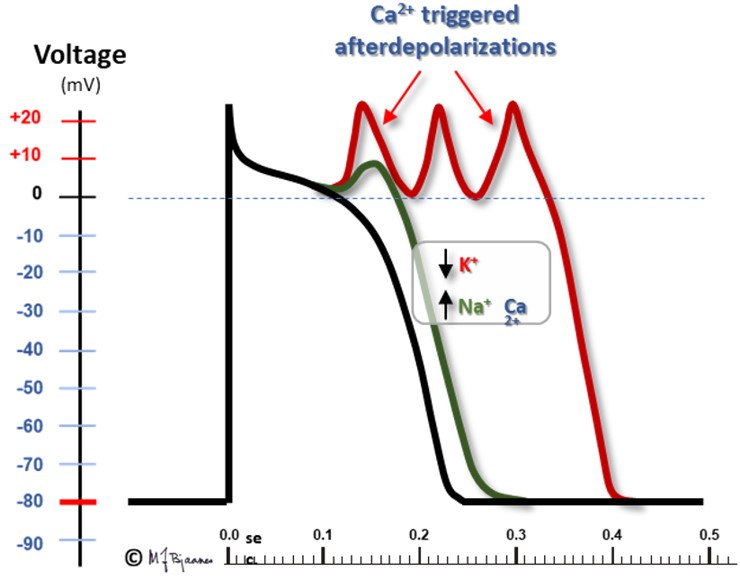
In some cases there is a genetic predisposition: in the inherited long QT syndrome mutations in the potassium channels delay the repolarization that terminates phase 2 of the action potential. Then systolic duration (QT interval) is prolonged, and more Ca2+ enters the cell. This activates the Na+/Ca2+ exchanger, resulting in a Na+ influx that causes a small depolarization (an afterpotential) that may trigger a VPB. This VPB may hit the relative refractory part of phase 3, and activate neighboring cells. A mutation in the sodium channels may inhibit their closing in phase 2, prolong this phase and result in a long QT-triggered VT.
Long QT time may also be provoked by drugs that inhibit the potassium channels. Antiarrhythmic drugs are often involved, but a long series of other drugs may also induce long QT and serious arrhythmias. A patient with an unexplained syncope should have an ECG recording, in particular if they take a drug that may prolong the QT interval. A list of such drugs can be found on https://crediblemeds.org/.
The illustration below presents a classical polymorphic VT.
Polymorphic VT with or without long QT time may also be acquired. Such potentially life threatening VTs are seen in myocardial ischemia, heart failure, electrolyte disturbances, intoxications and a series of other unstable conditions.

In ischemia/infarction too much Ca2+ will enter the cells, and arrhythmias may be triggered. Inherited defects in intracellular Ca2+-cycling may also elevate sarcoplasmatic Ca2+, and cause arrhythmias without prolongation of the QT interval.
Brugada syndrome
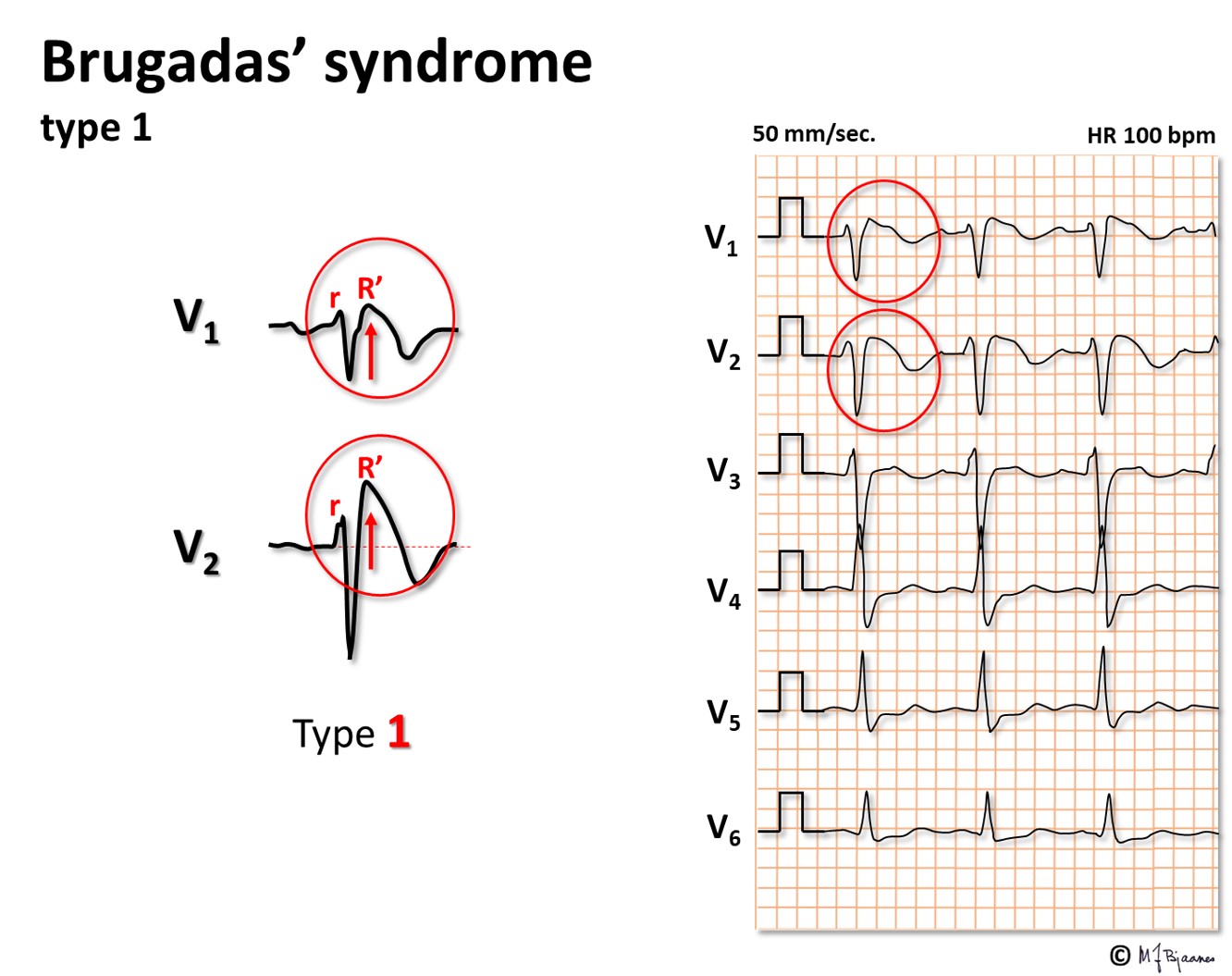
This is a rare inherited condition with sudden death caused by VT or ventricular fibrillation. Then, reduced function of the Na+ channels increases the repolarizing effect of the phase 1 current IKto. As IKto channels are most abundant in the epicard, there will be a potential difference between epi- and endocard in phase 2, as reflected by an elevated J point, especially in leads V1 and V2, and a coved ST segment elevation followed by negative T waves. The arrhythmia mechanism is complex, involving both triggered automatism and reentry. The illustrations below illustrate this tissue heterogeneity (however, extremely exaggerated):
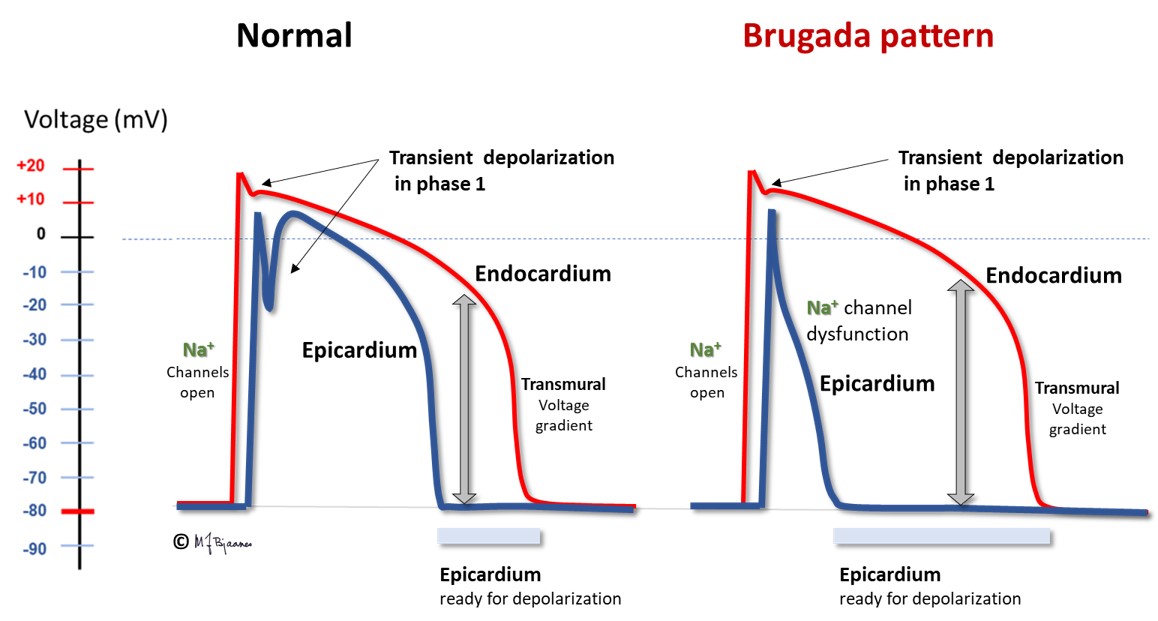
Several sodium channel mutations may be responsible for this pattern. The septal outflow area is the main contributor.
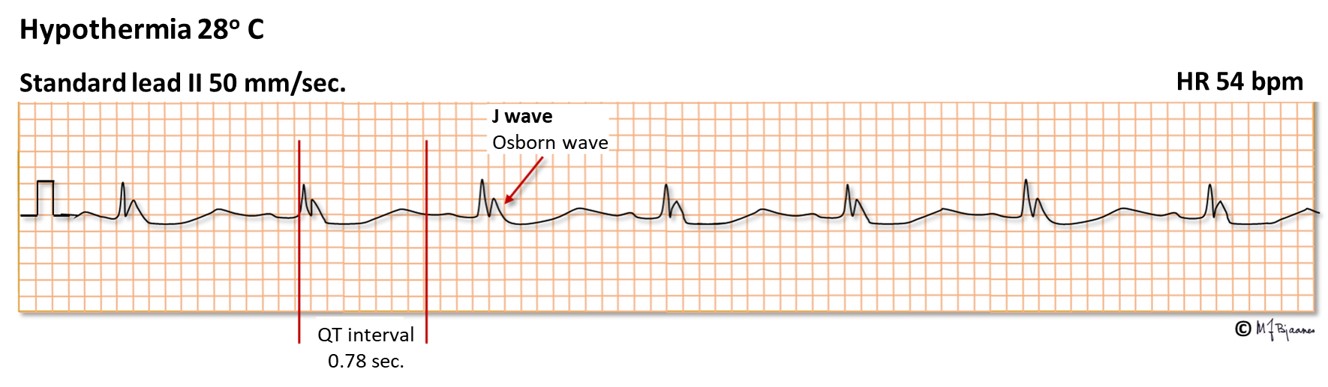
In hypothermia biological processes are, in general, retarded, and this also involves closing of ion channels. This results in bradycardia, prolongation of the PQ interval, a more marked phase 1 current (transient outward repolarizing potassium current), especially in epicard, and hence, a more marked J wave (see part 2), and the QT interval is prolonged. In addition, the ECG is also disturbed by noise signals from shivering muscles. The ECG changes appear at a core temperature around 32°C. When the potential difference between epi- and endocard increases, life-threatening phase 2 reentry arrhythmias may occur. At a temperature below 28° C the ECG may become flat. After 9 hours heart arrest, heart and lung resuscitation during slow rewarming was successful in a drowned young female who had a core temperature of 13.7°C, and she survived without sequelae.
Myocyte repolarization (phase 3, the T wave) is a vulnerable phase. A VPB that hits the T wave (R-on-T) may trigger a polymorphic ventricular tachycardia and VF in predisposed hearts, as in acute myocardial infarction, heart failure and electrolyte disturbances. For this reason, even a patient with a heart that «is too good to die» may suffer from sudden death in the course of even a small myocardial infarct. When the cardiac care units in hospitals were established in the 1960ies, the benefit was mainly the opportunity to convert VF immediately. The majority of these patients had R-on-T induced VF. The ascending part of the T wave is the most vulnerable. A VPB here may cause an afterdepolarization that triggers another VPB that conducts slowly to surrounding myocytes that join in, and the VT/VF series continues.
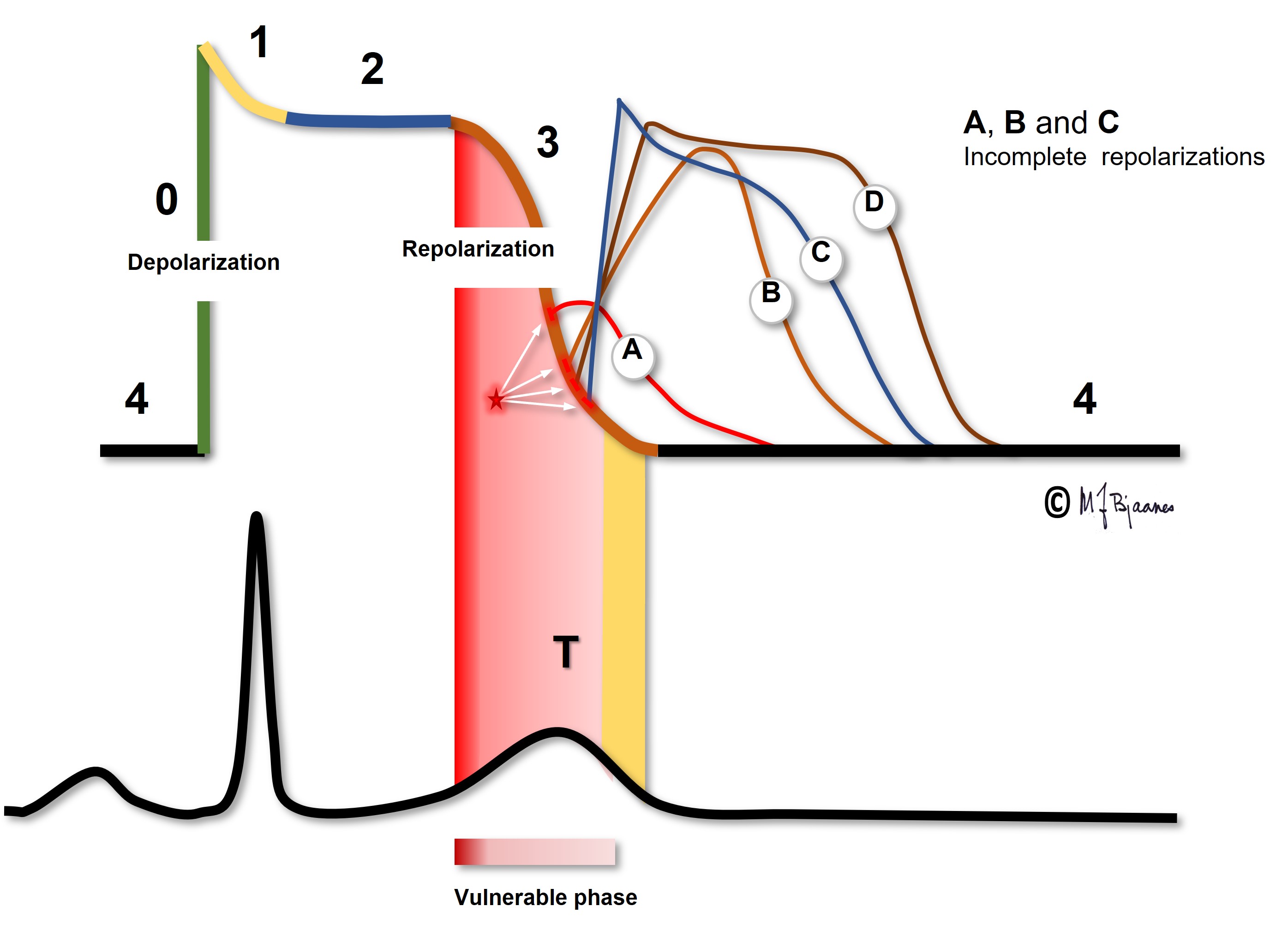
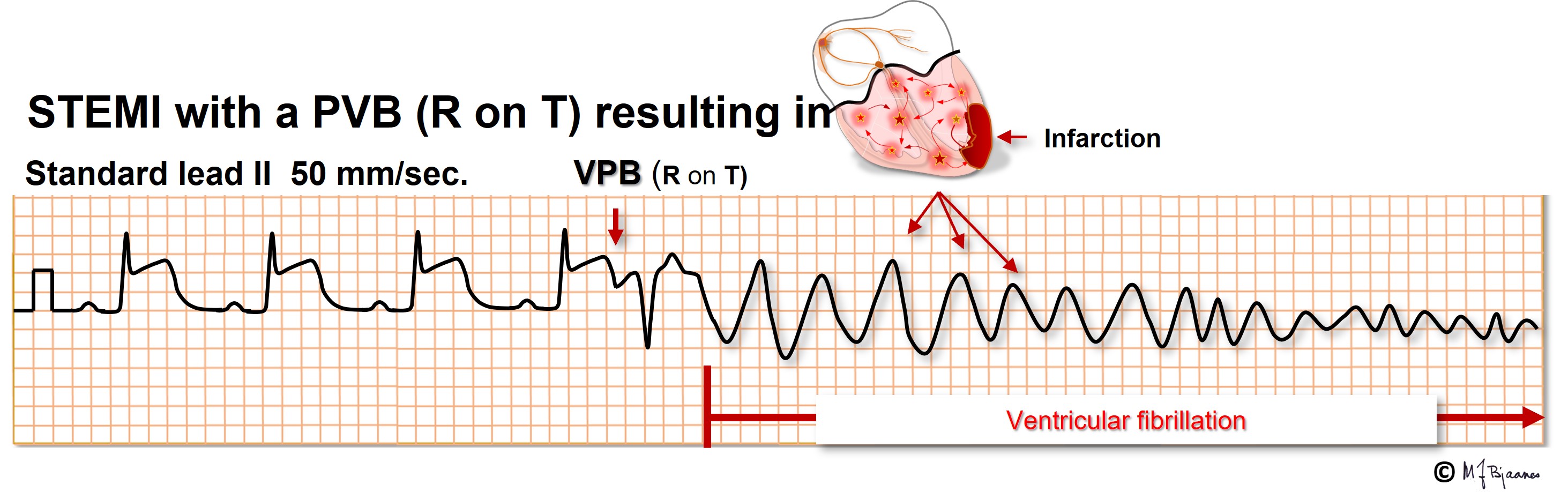
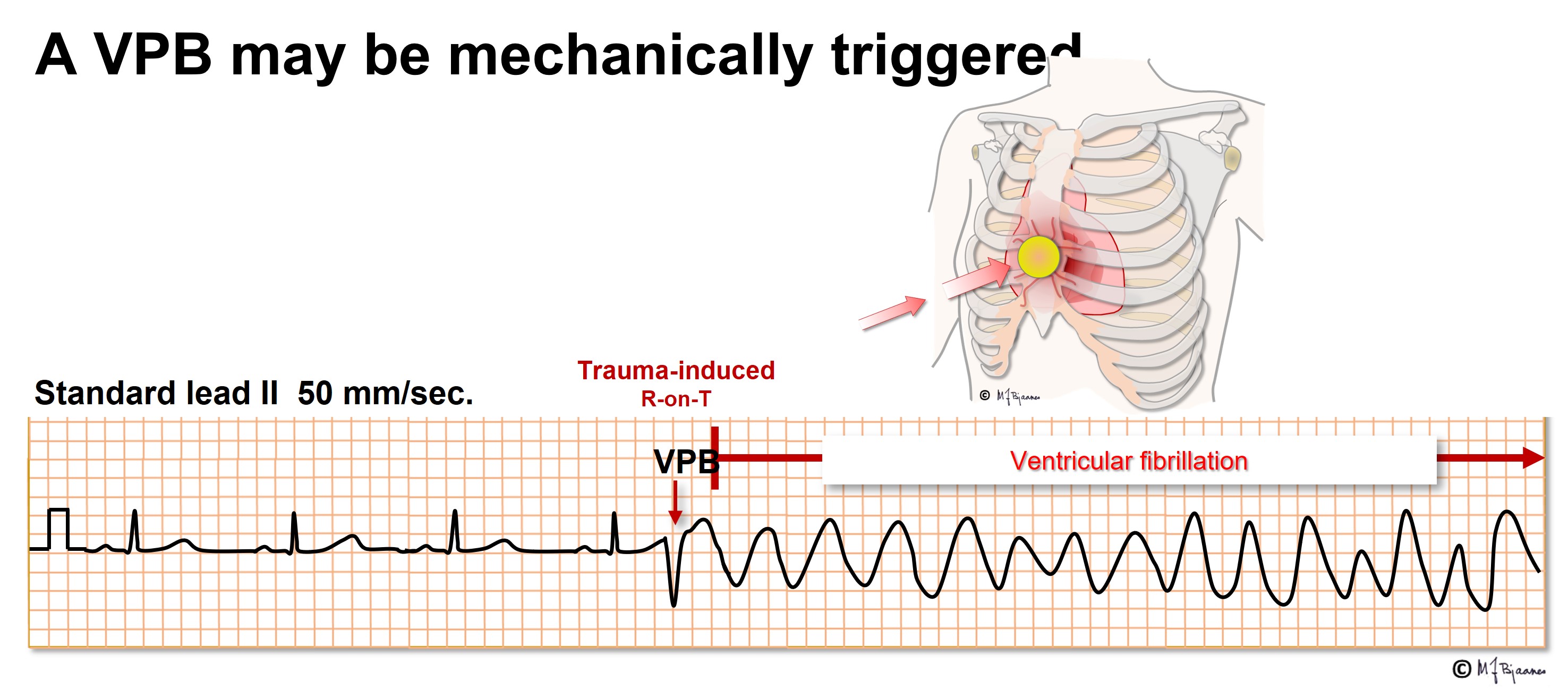
A VPB that hits this vulnerable zone may cause VF even in healthy persons. A hard blow to the chest, for instance from a baseball, may induce a VPB that causes VF, and if an electric current reaches the heart at the critical time window, again VF may result. If an implantable cardioverter/defibrillator is tested during implantation, a DC shock is given just in this window, it triggers VF and allows the device to prove its efficacy. For this reason elective DC cardioversion for atrial flutter, AF or VT, the DC shock should be given synchronously, that is, on the R peak to avoid hitting the T wave.
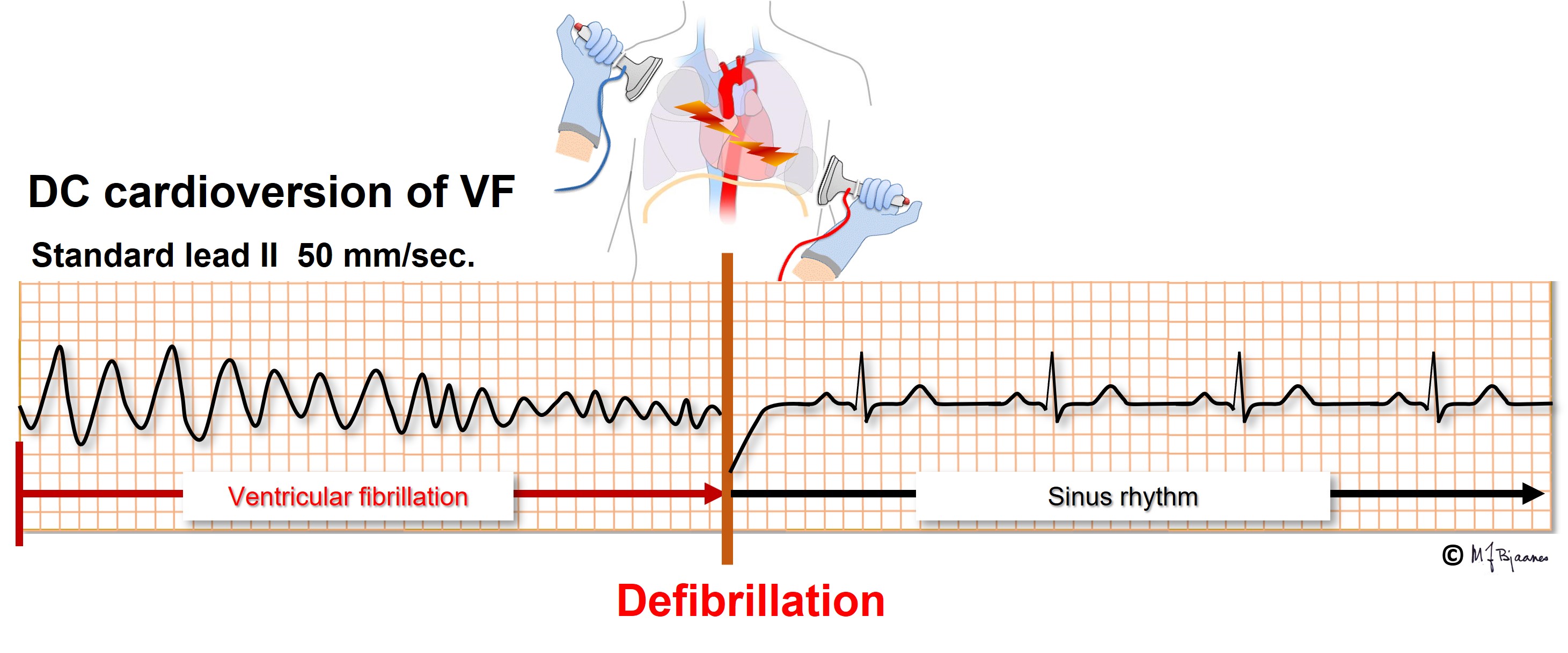
While a polymorphic VT spontaneously may terminate, VF requires DC cardioversion. By this procedure the entire heart is simultaneously depolarized, and during the pause that follows, the sinus node or another part of the conduction system may initiate the normal heart rhythm.
In 10-15 % of cardiac arrest cases, ventricular fibrillation is not a cause, but there is complete failure of the cardiac pacemaker/conduction system. Then attempts of cardioversion will fail because impulse generation is the problem. Mechanical stimulation by chest pounding or CPR is needed until either the patient recovers or an electrical device for stimulation is available: transcutaneous chest wall stimulation (Zoll pacemaker) or transvenous temporary pacing. Occasionally i.v. infusion of Isuprel is helpful.