In this part of the course you will learn about the resting membrane potential, the action potential, and how the cardiac anatomy and physiology contribute to the signals recorded by the electrocardiograph. The signals are modified in the machine before they are presented, often with a suggested interpretation.
The heart pump depends on electrical impulses.
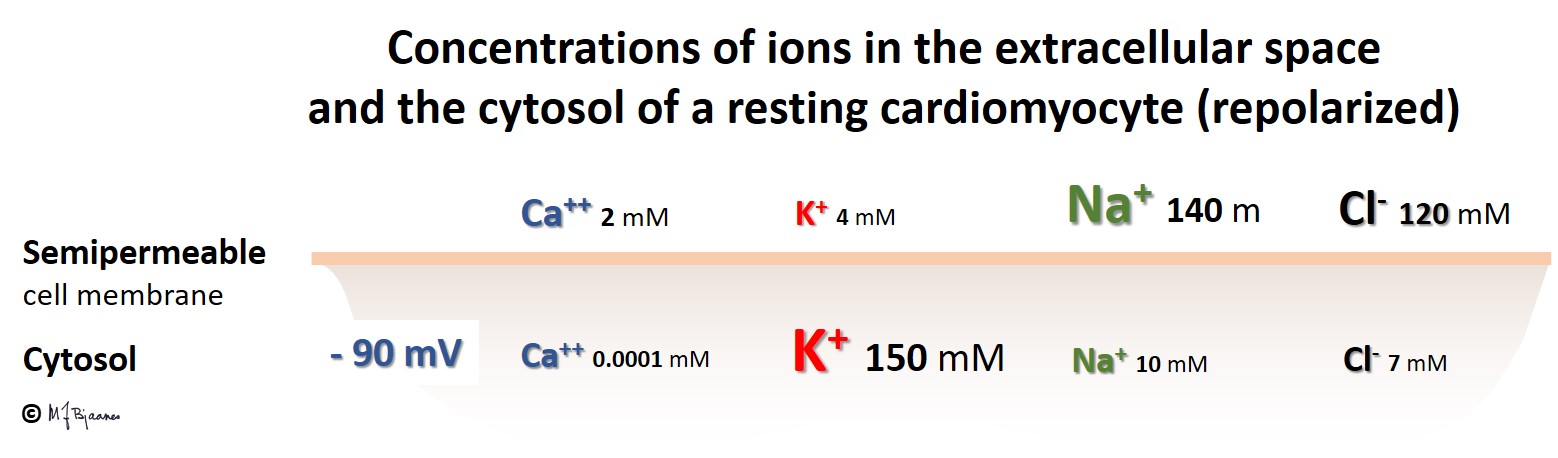
The prerequisite for electrical impulses is a surplus of negatively charged particles/ions inside the heart cells, and a surplus of positively charged particles/ions on the outside. This is the setting of a resting heart cell:

The negative charge within the cells is in part generated by negatively charged proteins, and in part because only the positively charged ions (K+) are able to leave the cell in its resting phase. The intracellular concentration of K+ (approx. 150 mM) is very much higher than the extracellular concentration (approx. 4 mM). This concentration gradient favors outward K+ diffusion, leaving a surplus of negatively charged ions inside the cell.
The difference in charge across the membrane (inside negative, outside positive) constitutes an energy source, i.e. an electrical potential that can be measured in volt. In diastole this potential is coined the resting membrane potential, and is around minus 90 mV in ventricular myocytes. In this phase the cell membrane is polarized.
The resting membrane potential depends on two important cell components:
Please turn on the speaker to hear doctor Stokke explain this animation.
Sodium
Electric currents in the heart are generated when a part of the cell membrane suddenly permits the inflow of positive ions (Na+) through large ion specific proteins called ion channels. These sodium channels open when the neighboring channels have a reduced membrane potential; the channels are voltage dependent. Since the outside Na+ concentration is much larger (approx. 140 mM) than inside the cells (approx. 10 mM), sodium channel opening initiates an inward sodium flush of ions. In a small area around each sodium channel, the membrane potential approaches zero – now the membrane is depolarized. This results in opening neighbor sodium channels, and the process rapidly spreads along the cell membrane.
Calcium
The cell membrane is also penetrated by other ion channels that react upon voltage change. Calcium channels open when sodium channel opening has reduced the membrane potential. Due to the very much higher Ca2+ concentration outside (approx. 2 mM), compared to inside (approx. 0.0001 mM), calcium will pour into the cells when the calcium channels open, adding to the membrane depolarization.
The cells store a high concentration of calcium in their sarcoplasmatic reticulum. The Ca2+ that has entered the cell, now binds to and opens the closed channels here (ryanodine receptors, RyR), and when open, they release large amounts of Ca2+ into cytosol.
Ca2+ in cytosol then binds to troponin C in the complex of contractile proteins, initiating the sliding movement of actin and myosin that results in sarcomere contraction.
Potassium
The opening of sodium and calcium channels in the membrane thus results in an influx of positively charged ions. However, these channels are only open for a brief period of time, and further, the depolarization result in opening of potassium channels that permits outward potassium current that reduces the dominance of the positively charged ions. Soon these channels close, and also, the depolarization leads to opening the potassium channels that repolarize the cells, as the outward potassium current counteracts the depolarizing effect of Na+ and Ca2+ influx. During repolarization the sodium channels close.
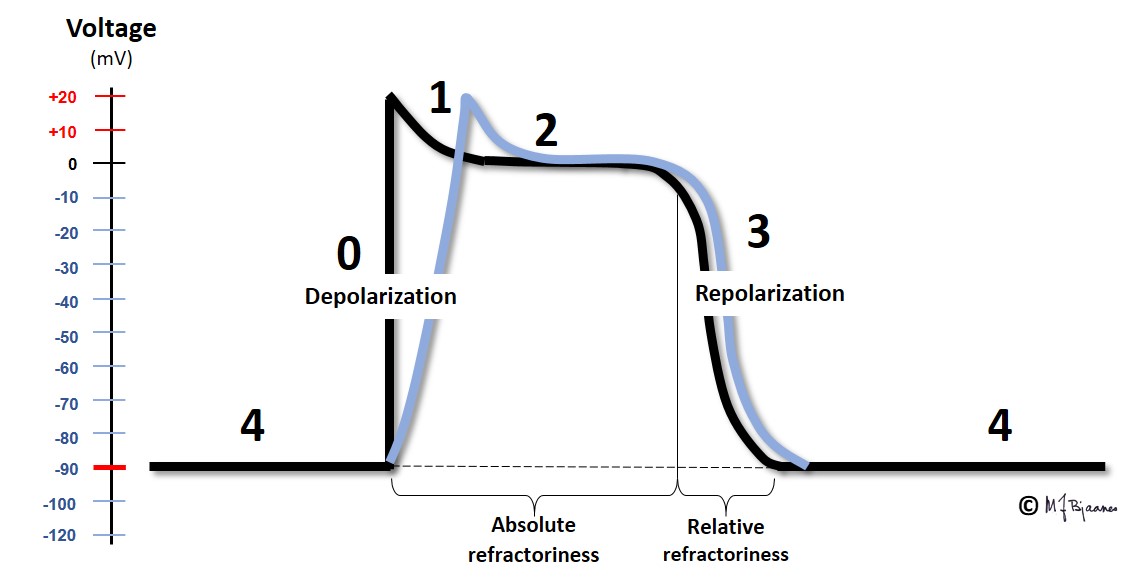
The sequence of ion movements follows the same pattern for every heart beat, and this variation in the membrane potential, the action potential, can be measured. The description given so far, holds for the majority of the ventricular and atrial myocytes:
We start with a polarized cell in diastole. The inward Na+ current depolarizes the cell (phase 0). This results in opening the calcium channels, and also a number of potassium channels with different properties. One of the latter channels open early, resulting in a slight repolarization (phase 1), from approximately +30 mV to 0 mV. This repolarization, however, is balanced by a depolarizing inward Ca2+ flux, and thus the membrane potential is stable for a period of time, the plateau of the action potential (phase 2). After some delay, more potassium channels open, while the calcium cannels close. Outward K+ current then dominates, and repolarizes the cell membrane (phase 3). A last the cell membrane reaches its resting potential, and then the Na+/K+-pump expels N++ from the cell in exchange with K+ that is pumped into the cell (phase 4, diastole).
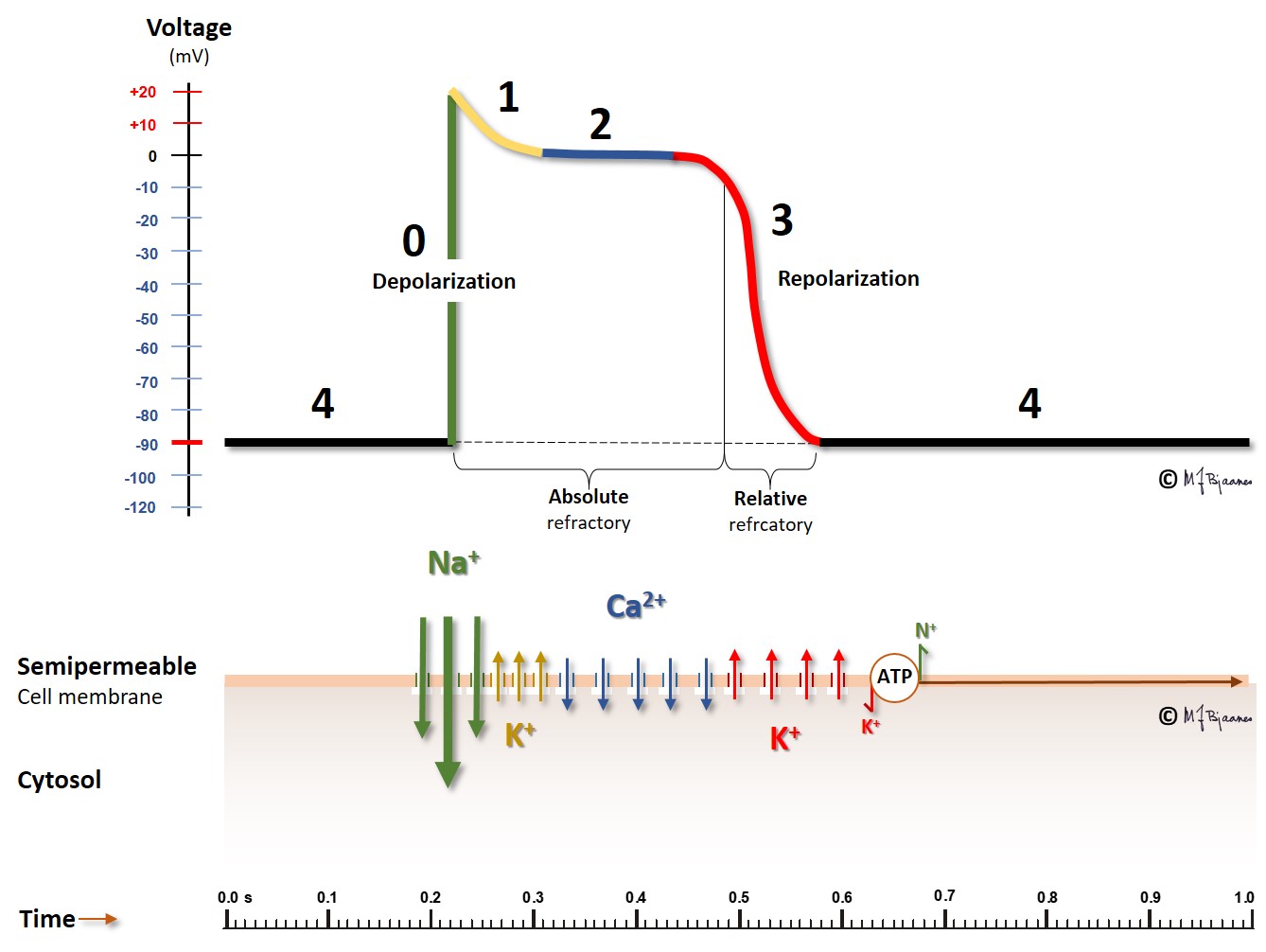
This figure demonstrates the action potential from a myocyte in the ventricles or atria. In the pacemaker cells, however, the pattern is much different; see the figure below:
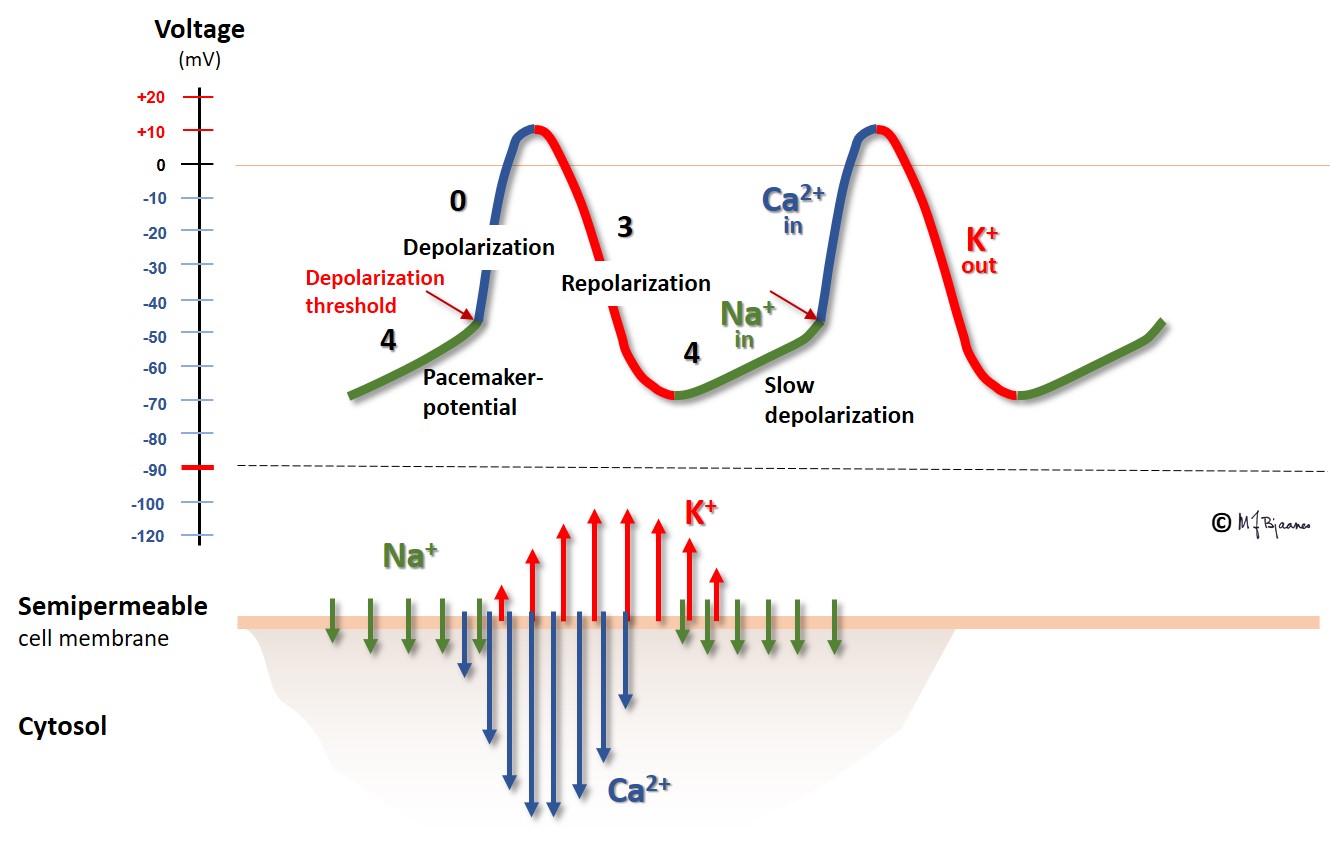
Another cell type, the pacemaker cells, have spontaneous depolarization in phase 4, as their membrane potential is slowly reduced in diastole by the funny current (If) that permits positively charged ions to enter the negatively hyperpolarized cells, and with contribution from a simultaneous inward Ca2+ current, the membrane potential reaches a firing threshold and triggers the rapid depolarization of an action potential. The name funny current was chosen because hyperpolarization opens this channel, at variance from other channels that are opened by depolarization. You should note that the action potential of the pacemaker cells lack the phases 1 and 2; they have an immediate transition from depolarization to repolarization.
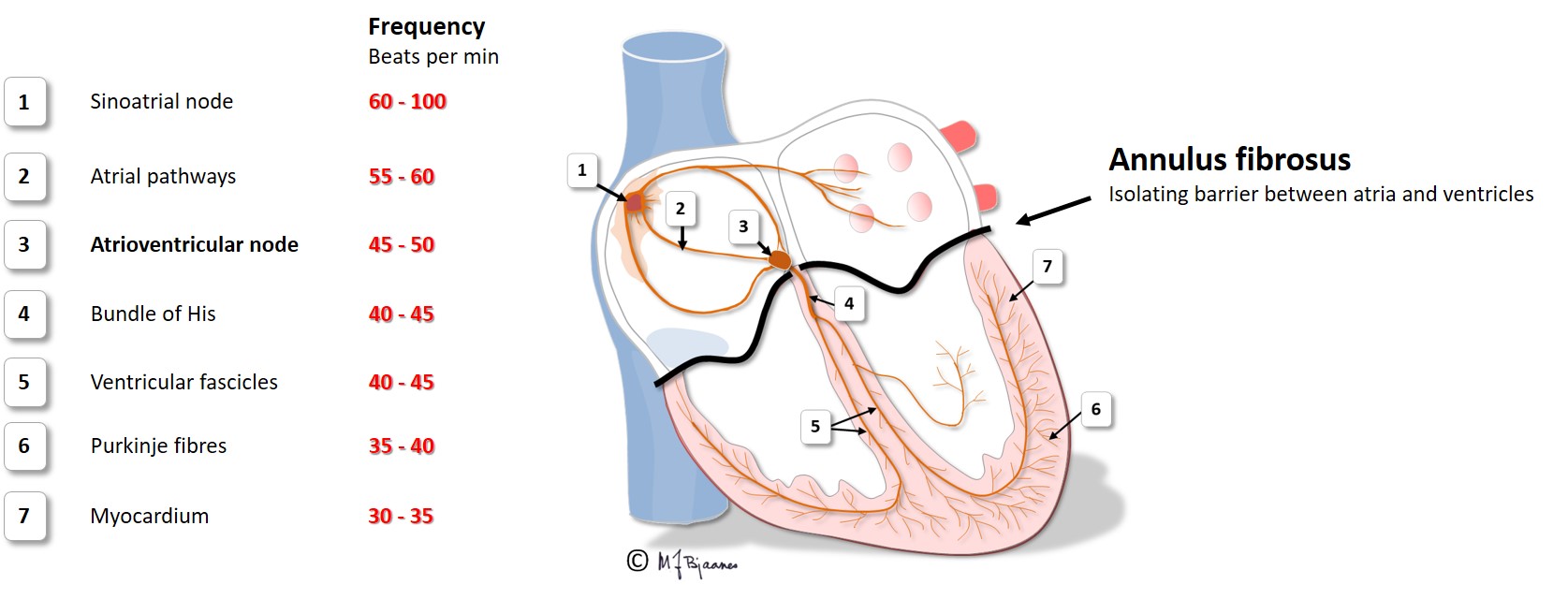
The conduction system of the heart has three main functions:
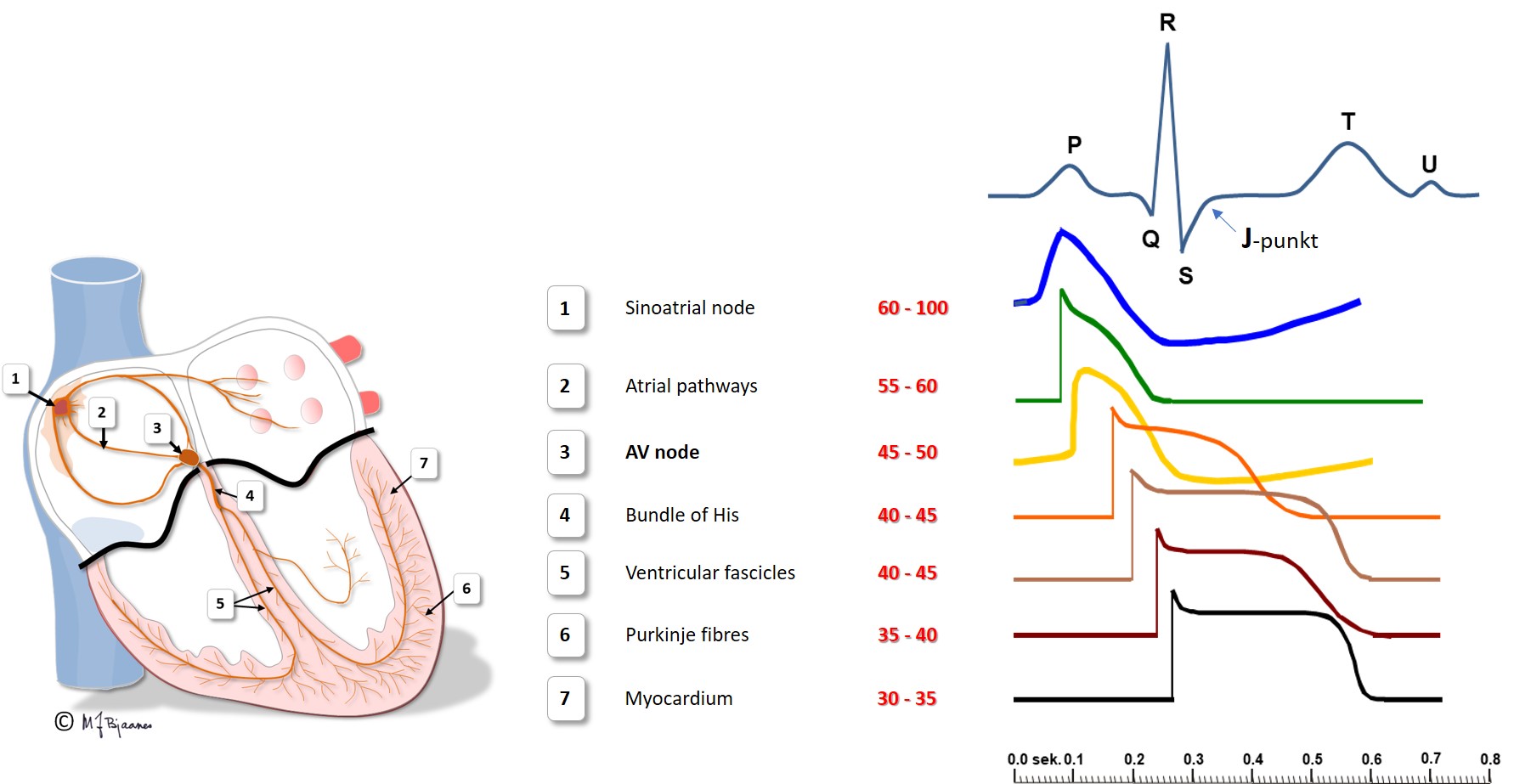
The pacemaker cells in the sinus and atrio-ventricular node (AV node) have little cytoplasma and a large nucleus. Their actin and myosin contents are small, but the cells have a rich innervation and generate impulses. The fastest pacemaker cells determine the heart rate, and usually such cells are found near the roof of the right atrium, in the sinus node.
The conduction cells in the His’ bundle, the bundle branches and purkinje fibres are thicker, elongated and fast conducting. Their morphology reveals that contraction cannot be their main function. Also they have pacemaker function, but they fire at a much slower rate compared to the cells of the sinus and AV node.
The sinus node normally has the fastest pacemaker cells, and determines the heart rate. The pacemaker cells usually have a rich blood supply and dense innervation. The fastest pacemaker cells are found high up in the heart, in the sinus node, but if they fail, or have been slowed down too much, cells from lower parts of the conduction system may take over. Occasionally there are ectopic pacemaker cells outside the sinus node; in the atria or close to the orifice of the venae cavae or pulmonary veins, and such cells may generate premature beats (extrasystoles).
If the resting membrane potential is weakened, phase 0 of the action potential becomes less steep (lower dV/dt), for instance, if the K+ concentration is high outside the cell. This occurs in ischemia, where the energy demanding ion pumps fail. The same happens when the inward Na+ current in phase 0 is inhibited by drugs that block the Na+-channels.
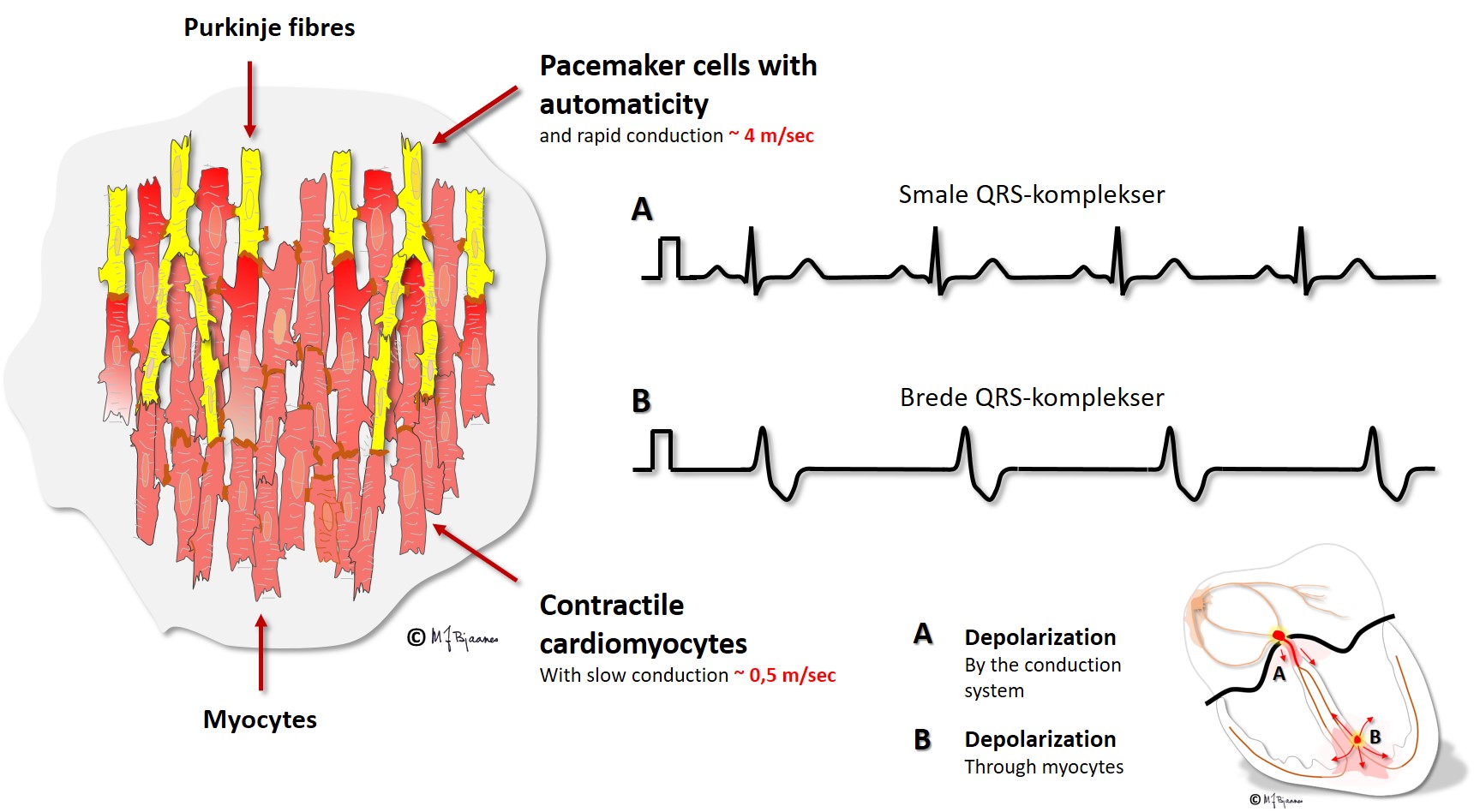
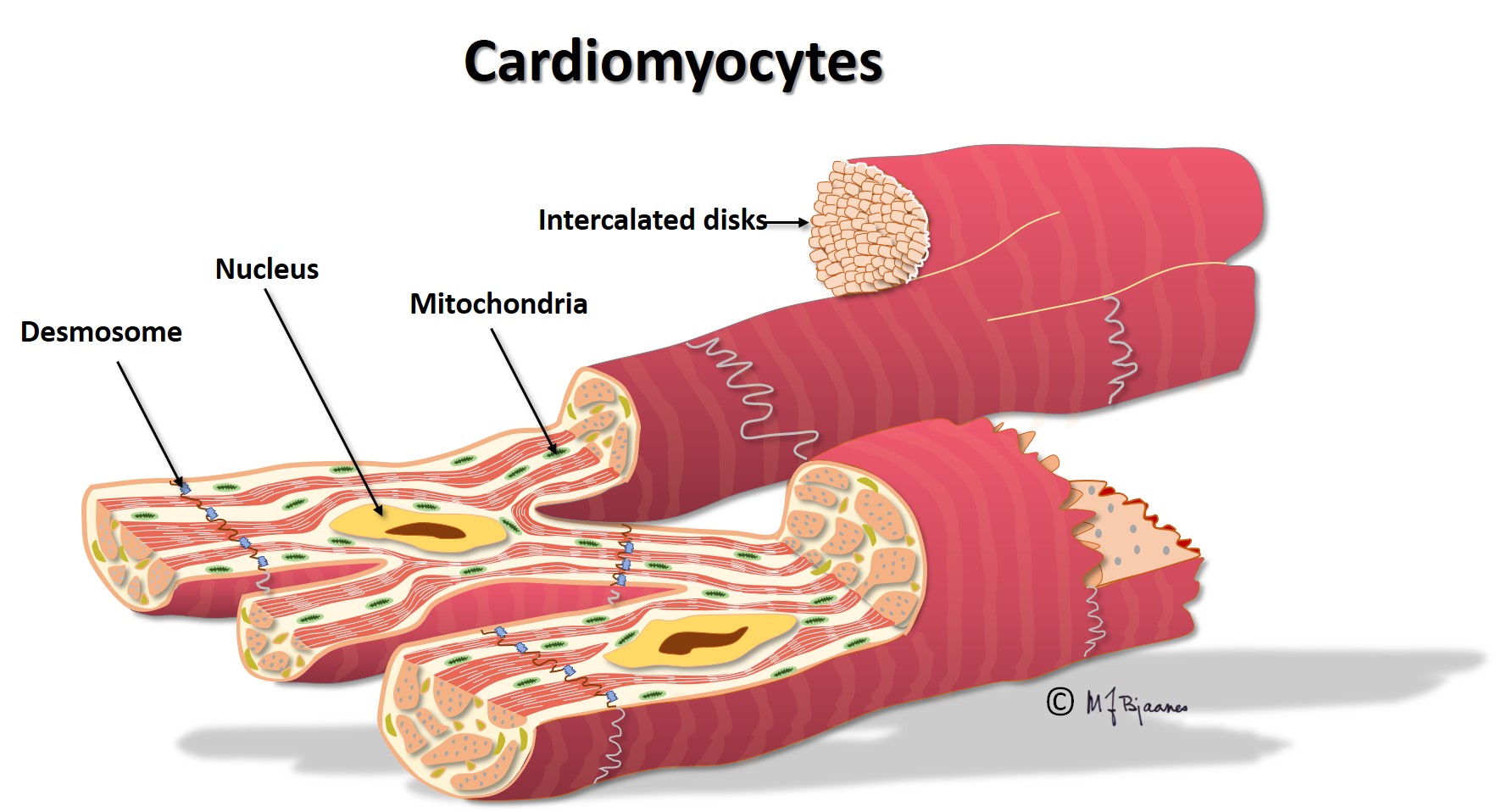
Different types of heart cells and the way they are organized, facilitate efficient cardiac work at a lower energy cost. The wave of depolarization rapidly flows along the cell, but is delayed by the intercalated discs. (This is described more in detail in the section on cardiomyocytes). For this reason impulse conduction is faster in cardiac muscle with longitudinal, compared to transversal fiber orientation. The fastest conduction is found in the specialized elongated conduction cells. The animation below shows the normal cardiac conduction:

In the ECG depolarization presents as a QRS complex; narrow when the heart chambers are activated through a normal conduction system, but broad if the impulses are spread from myocyte to myocyte outside the conduction system.
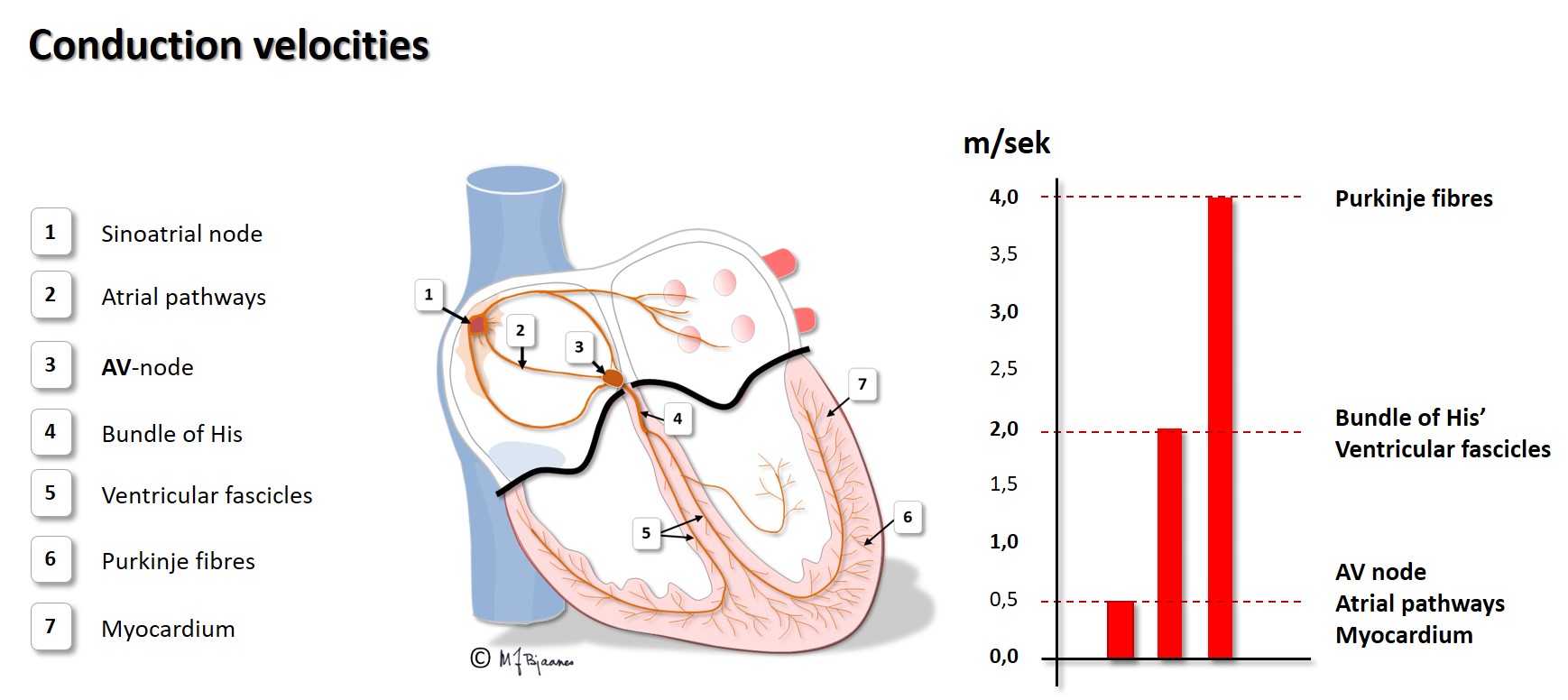
The speed of conduction in the various parts of the heart is shown below:
Emptying the atria is facilitated by the initial contraction wave that starts high up and then moves downward to the ventricular inlet valves. The AV node blocks impulses that are too close, reducing the risk of too rapid ventricular contraction in case the atria should run away fast, as for instance, in atrial fibrillation where the AV node is bombarded by impulses. Also the normal atrial impulses that reach the AV node, are delayed there, providing time for the ventricles to receive sufficient filling of blood. When the atria contract late in ventricular diastole, the additional atrial kick increases ventricular filling, and the increased wall stress (preload) augments the contractile forces of the ventricles. The timing of atrial and ventricular contraction is thus important for the efficacy of the heart pump.
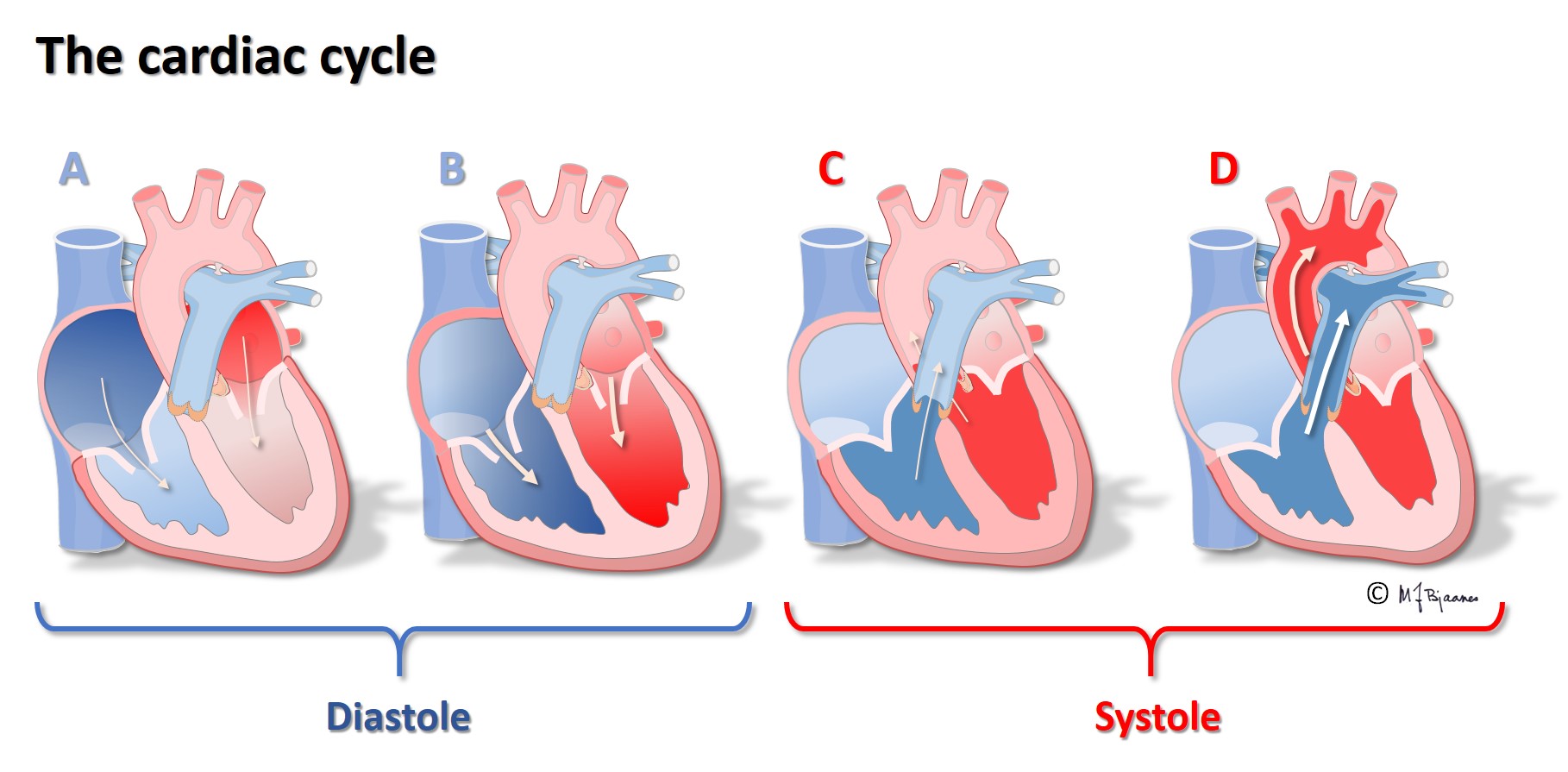
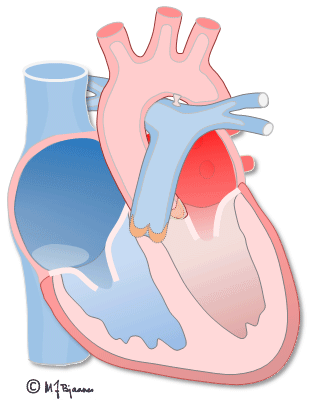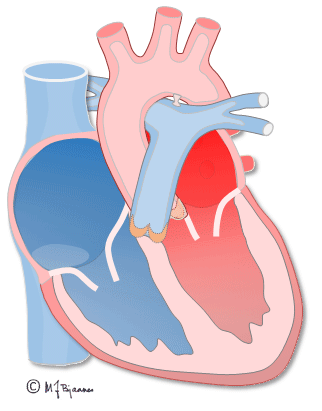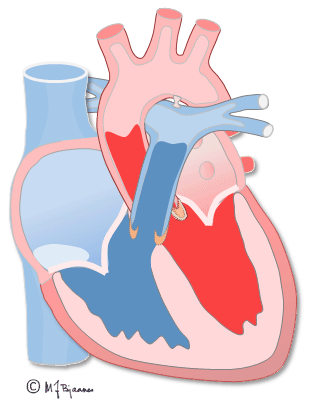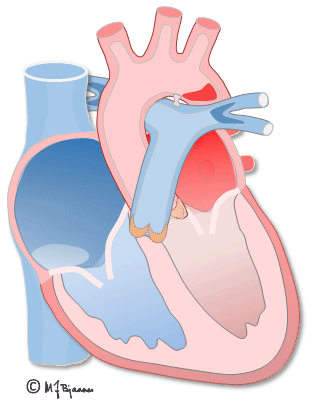
The first ventricular activation takes place high up in the septum, which thus is straightened and stabilized. The further impulses are spread by the specialized conduction system, and rapidly activate both ventricles (see animation above). The simultaneous contraction of the two ventricles facititates efficient cardiac ejection of blood.
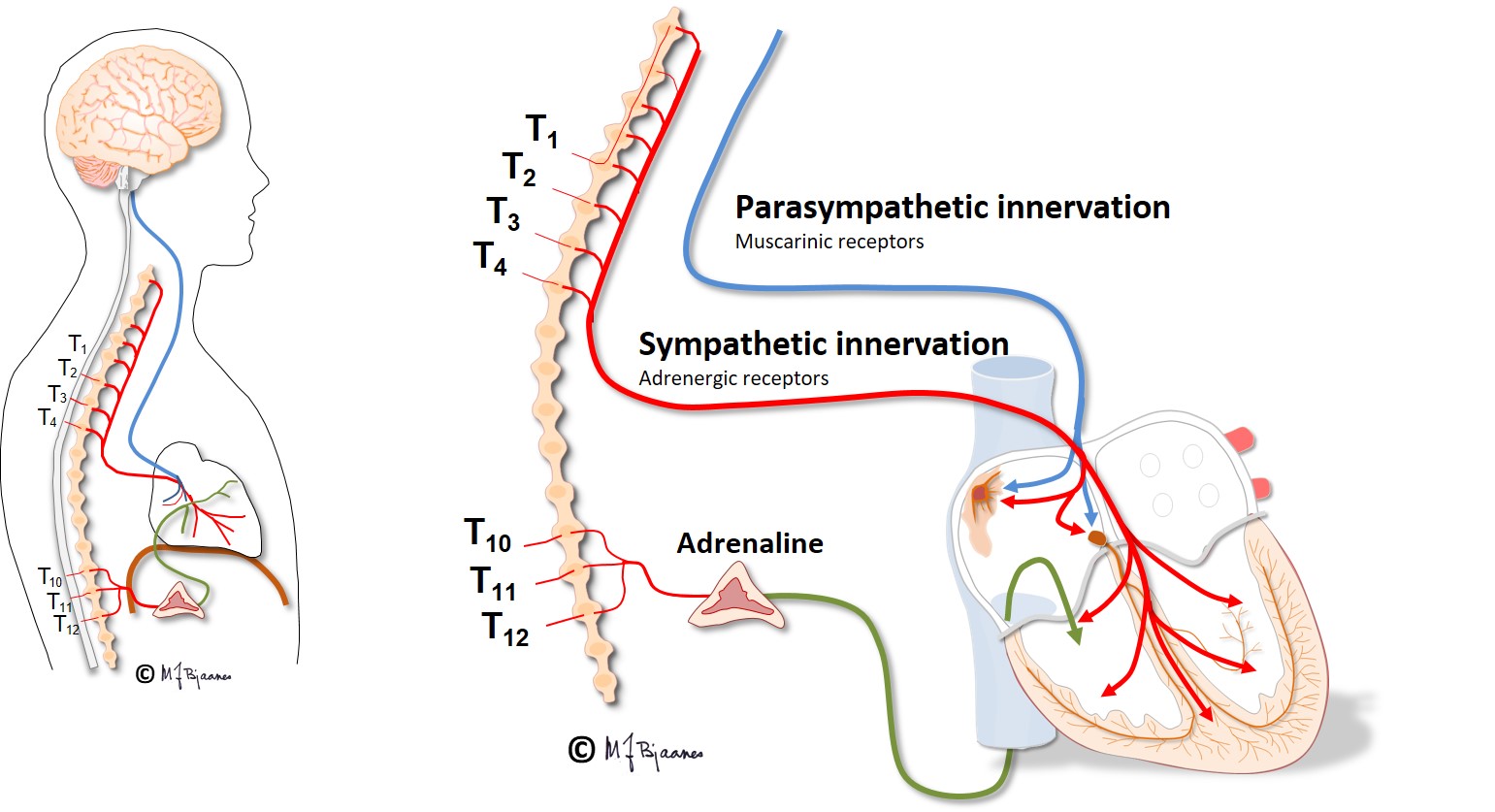
The cardiac impulse and conduction system is richly innervated by sympathetic and parasympathetic nerves, especially to the sinus and AV node. Under pharmacological autonomic blockade or cardiac denervation (for instance in the transplanted heart), the adult heart will beat around 100 beats per min (bpm), illustrating that at rest, there is vagal control of the heart rate. In high spinal cord injury, vagal control of the heart rate is preserved, but the sympathetic nerves are disconnected, causing slower heart rate at rest, and especially so, at physical exercise.
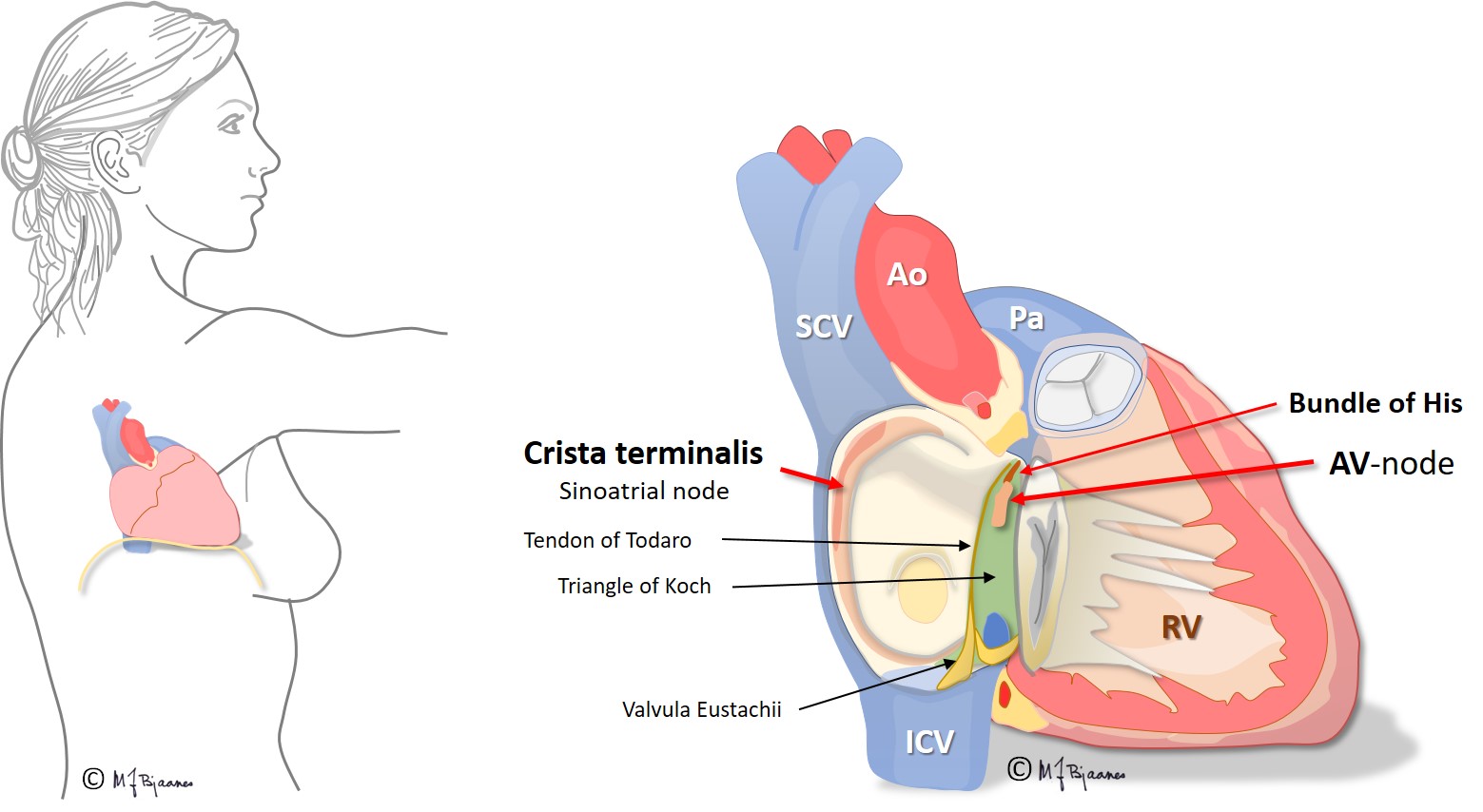
The sinus node was discovered by the medical student Martin Flack, who dissected a mole under the supervision of Arthur Keith (1907). It is a banana shaped flat right atrial structure that stretches from the high atrium to the midwall: 1-3 cm long, 0.5 cm broad and 0.1 cm thick.
The sinus node has its blood supply from the first side branch of the right coronary artery. If a patient with acute myocardial infarction presents with sinus node failure, a proximal occlusion of the right coronary artery (RCA) is likely. There is rich autonomic innervation of this node, both from vagus and sympathicus. The intrinsic rhythm of the heart is around 100 bpm, but vagus usually retards the resting heart rate down to 50-80 bpm. There are stretch receptors in the atrial walls, and when right atrial filling increases, as during inspiration, afferent inhibitory signals from the vagus nerve reach the vagal nucleus, reducing the efferent inhibitory signals, and the heart rate increases. This mechanism is responsible for the respiratory variations in heart rate. Heart rate variability thus reflects the balance in the autonomic nerve system. (This is described further in Part 4). Children, the young and athletes often have great heart rate variability, whereas the vagal activity is weaker, with faster and more regular heart beats among the untrained and the elderly, and the respiratory variations may be abolished in a patient with heart disease.
Inappropriate sinus tachycardia is an infrequent disease, most often seen in younger females, and the heart then beats too fast at rest, and even more so during exercise. When drug treatment is prescribed, the first choice is to retard the pacemaker cells with a beta-adrenergic receptor blocker, then an L-type calcium antagonist is tried, and finally, ivabradine, an inhibitor of the funny current, If, is tried. In rare cases, even destruction of the fastest pacemaker cells has been tried by heating or freezing applied by a catheter introduced through a vein (catheter ablation). A sick sinus node may stimulate too slowly, and if its firing stops, even cause fainting spells if rescuing escape rhythm is not provided by pacemaker cells from further down the conduction system. This is discussed in the arrhythmia part (part 4).
Impulse conduction in the atria is not transmitted through specialized conducting cells, but occurs faster where the muscle cells are elongated and oriented in parallel as «bundles». Such a roof bundle connects the atria, and bundles from both atria lead to the region around the AV node. The heart surgeons try to avoid transsection of these bundles, to avoid slow impulse propagation (manifested as broad P waves) that promotes atrial heart rhythm problems.
In enlarged atria with increased connective tissue or inflammation of their walls, impulse propagation takes more time, and that increases the risk of atrial circuit currents that may cause and maintain atrial fibrillation (more on this in parts 2 and 4).
The AV node is a complex structure on the atrial side, in the middle of the heart. It was first described by Sunao Tawara, a Japanese physician who then worked in Germany (1905).
The AV node receives atrial impulses, modulate them and transport them to the His’ bundle. Its function is reflected in the ECG as the time from onset of atrial depolarization (P wave) to the onset of ventricular depolarization (QRS).
The AV node is located between the aortic, mitral and tricuspid valves (see illustration), onto the electrically isolating annulus fibrosus, the connective tissue that holds all cardiac valves. Proximity to the valves makes the AV node vulnerable to injury from valve inflammation and surgery. The position in the center of the heart, between the atria and ventricles, and between the right and left side of the heart, exposes the node to mechanical wear and tear stress, often resulting in fibrosis and calcium deposits. Such an injury may increase the conduction time across the node, and even block impulse passage. If conduction across the AV node fails, transient or permanent cardiac arrest will occur, causing syncope, or in the latter case, death from asystole if no escape rhythm appears from cells further down in the conduction system.
The AV node has some fast conducting areas (superior, anterior) and other areas with slower conduction (inferior, posterior), called the fast and the slow pathway, respectively. Their refractory periods differ: the fast conducting cells need a longer rest before they can conduct the next beat. The two pathways are separated by a core of no- or very slow-connecting tissue, and they have common entry and exit. Occasionally this structure permits circuit movement of the depolarizing impulses in the area, like a car never leaving a round-about. When the depolarization front is high up in the node, the atria are retrogradely activated, and when low, the His’ bundle is activated. The patients experience sudden onset (and termination) of tachycardia (paroxysmal supraventricular tachycardia, further discussed in part 4, arrhythmias).
The cells of the AV node are organized as a mesh, and this structure delays impulse propagation, permitting efficient heart work because the ventricles do not contract before they are properly filled. As mentioned above, the AV node also blocks an impulse that is too close to the previous beat. Conducted rhythm seldom is faster than 240 bpm. The AV node has a rich innervation. The vagal nerve reduces conduction velocity, whereas sympathetic stimulation facilitates (see illustration below).

In the wall of the common carotid artery, cells in the carotid sinus monitor blood pressure. If the precerebral blood pressure is too high, inhibitory impulses are transmitted through the vagus nerve, reducing blood pressure, impulse generation in the sinus node and impulse conduction in the AV node, further discussed in part 4. This is a useful mechanism for brain protection. The carotid sinus may further be useful in patients with supraventricular tachycardia caused by reentry. Then vagal stimulation may block one beat and thus stop the tachycardia. The patient is grateful and the doctor happy when the «magical finger» presses the neck and terminates the attack (more in part 4). On other occasions the carotid reflex is unwanted: Elderly people may have a hypersensitive carotid sensor, and a tight collar or a vigorous turn of the head may inhibit either the sinus node, the AV node, both, or causing vasodilation or transient conduction block, resulting in a dizzy spell or fainting.
His’ bundle is a thin «cable» that crosses the non-conducting annulus fibrosus, and connects the AV node to the right and left bundle branches. It was first described by the Swiss professor Wilhelm His when in Berlin in 1893. His’ bundle is normally the only electrical bridge through the connective tissue that isolates atria from the ventricles.
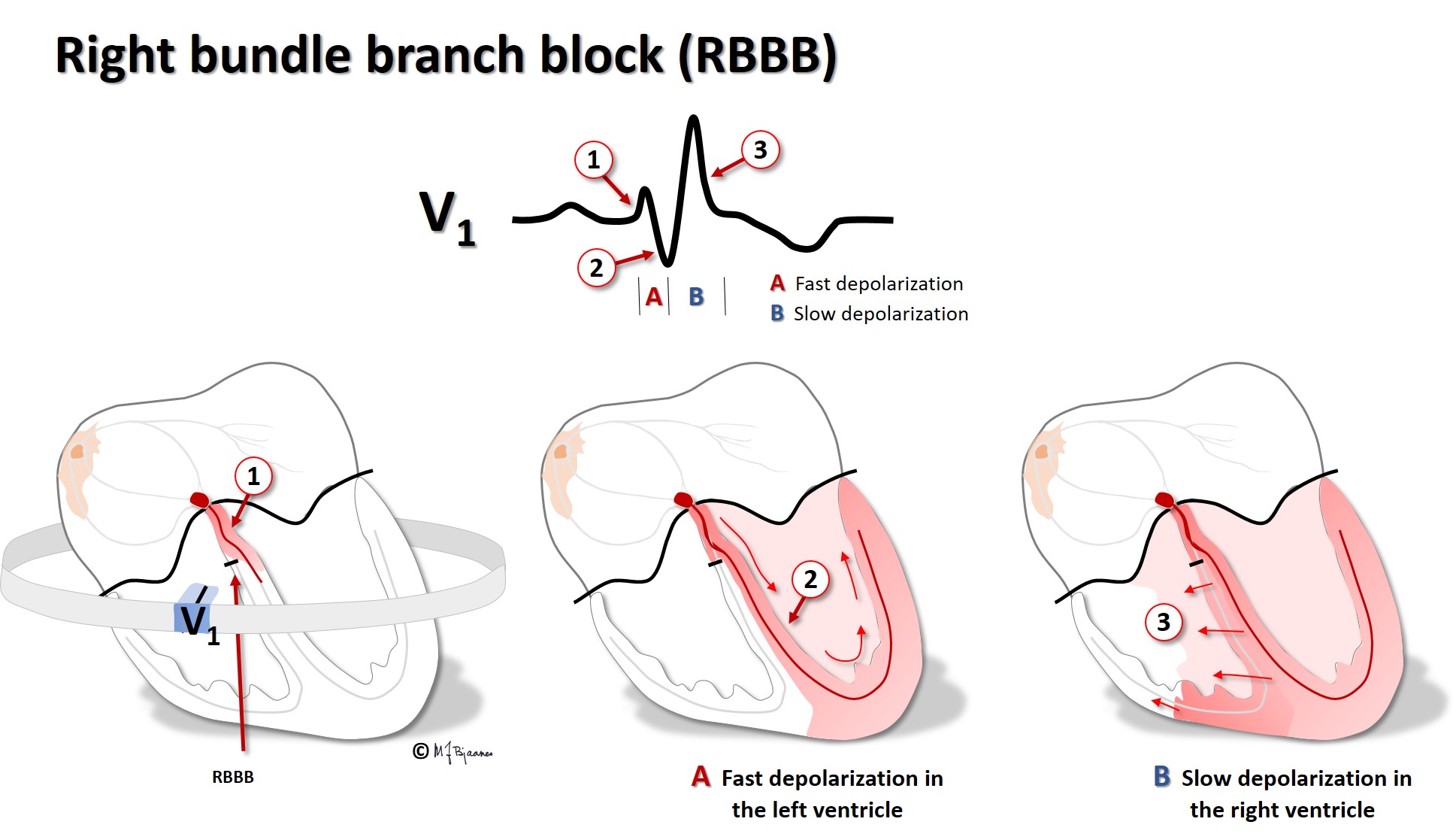
The bundles have got fast conducting myocytes, and also pacemaker cells. If an atrial impulse is stopped in the AV node, the bundle of His may generate a normal, albeit slower escape rhythm. His’ bundle block may cause cardiac arrest if no escape rhythm arises from the bundle branches or ventricles. First the right bundle enters its ventricle as a distinct cable that further down spreads out in the subendocardiumium. Injury of a small area in the bundle thus may block all right-sided conduction (right bundle branch block).
The left bundle divides into two (occasionally three) main branches (fascicles) high up in the septum, and they divide into smaller branches in a wide, fan shaped area. A complete left bundle branch block may thus reveal injury of a large muscle mass.
In bundle branch block one of the two main electrical connections between the bundle of His and the ventricles is disrupted, and myocytes on the affected side must be activated by impulses that traverse across the muscle fibers of the first activated ventricle. This takes time (the QRS complex broadens), and the chambers do not contract simultaneously. The septum will then flutter, and the heart work becomes less efficient.
In the left bundle, a so-called hemiblock may occur, either in the anterior or posterior fascicles. Then the activation time of the left ventricle is modestly prolonged, and the spread of activation will be abnormal (a broader QRS with an abnormal axis).
The fascicles further divide into purkinje fibres that spread around the entire subendocardium.
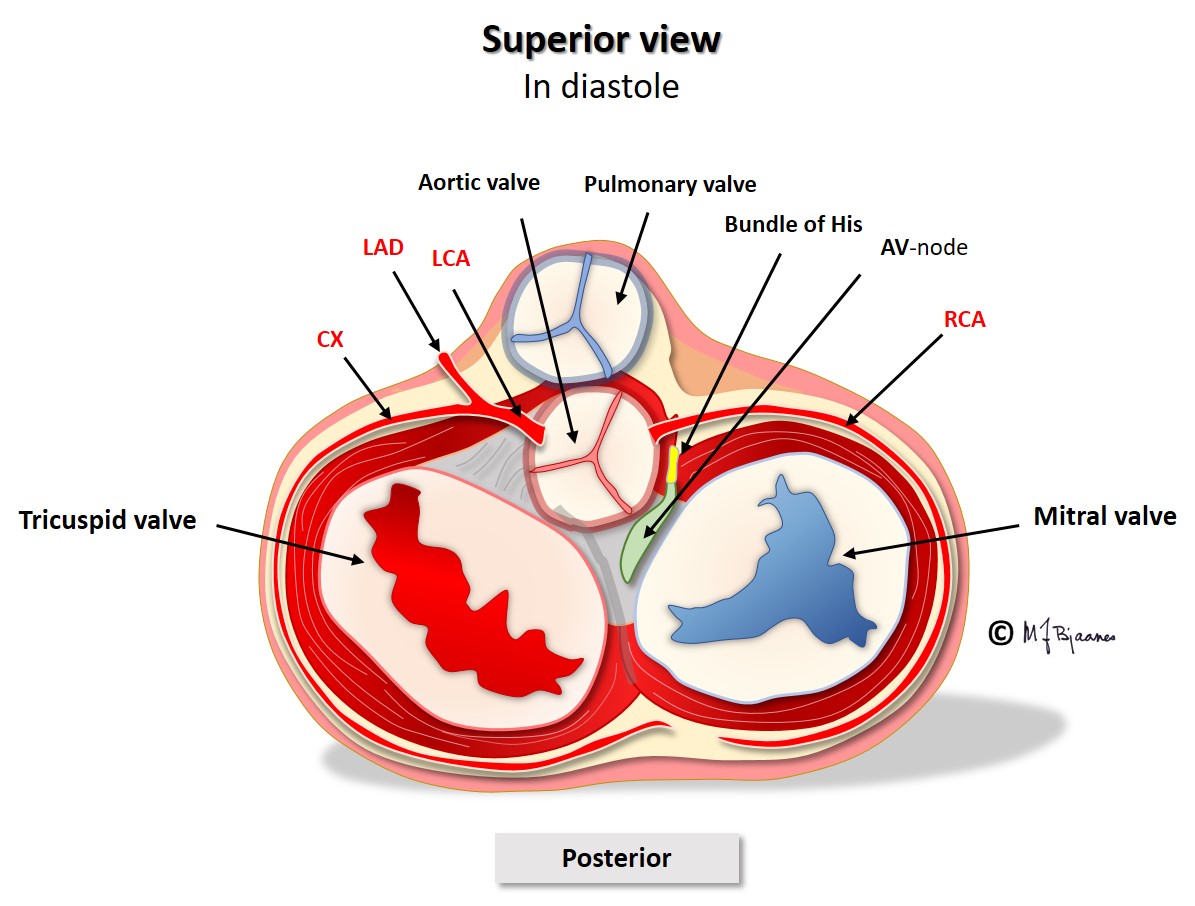
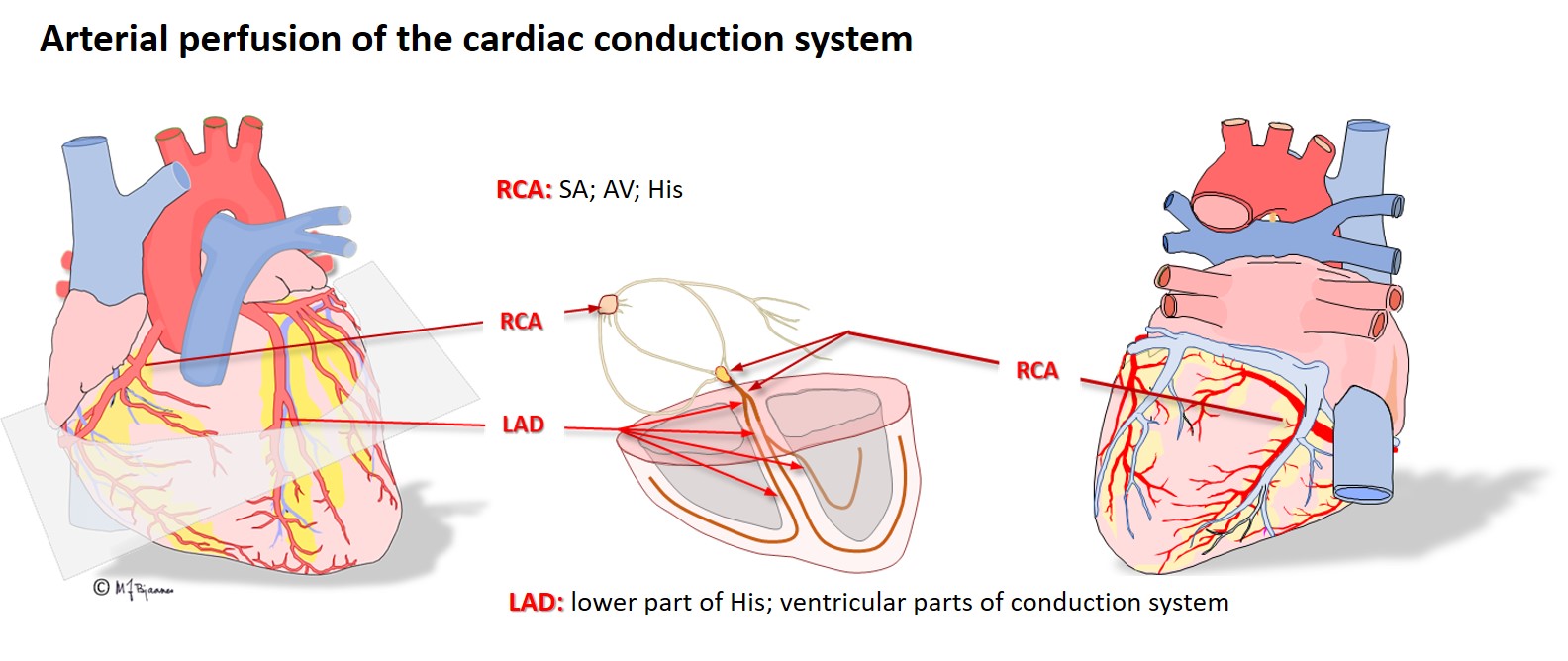
The right coronary artery (RCA) provides blood to the sinus and the AV node. If this artery occludes and causes an infarction, atrial impulse generation and conduction is frequently affected. The left anterior descending artery (LAD) nourishes the whole left bundle branch area, whereas the His’ bundle and the right bundle are supported by both the left and right coronary artery.
These were described by Jan Evangelista Purkyne in 1839. He was a professor of physiology in Breslau (now Wroslaw, Poland). He was a friend of both Goethe and Schiller, and mastered 13 languages (Wikipedia).
The purkinje fibres are organized in a subendokardial network in both ventricles, and secure a rapid spread of impulses to all the myocard. The cells are both broader and longer than the regular myocytes, and have fewer myofibrils and much mitochondria. Pacemaker cells are also found among them.

The regular myocytes in the atria and ventricles can lead impulses, but not generate them, at variance from cells in the conduction system. The myocytes are large elongated cells with small nuclei, much cytoplasma, mitochondria and myofibrils, and connect to each other mainly end-to-end, but also side-to-side. Thus they are able to work simultaneously, strongly, and continuously.
The myocytes are fast conductors of longitudinal impulses, and their frequent branching facilitates impulse spreading. Transversal impulse transport across the muscle is, however, slow, since delays occur at the intercalated disks. Some patients have genetic errors in proteins of the intercalated disks, and then impulse spreading is slow and may spin around in reentry circuits that may cause life-threatening fast heart rates (more in part 4). The action potential of the endocardiumial myocytes differs slightly from that of epicardiumial cells, and this influences the ECG (see later on).
The purkinje fibres are found just underneath the endocardium. Hence, the depolarization and contraction of the cardiac wall starts in subendocardium, and rapidly spreads outward to the epicardium.
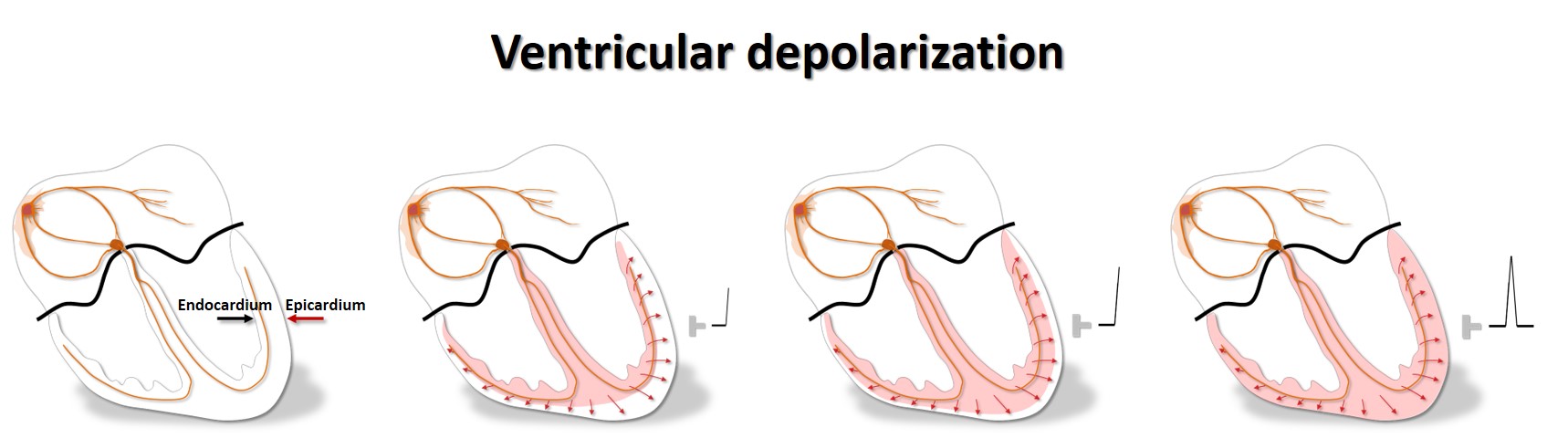
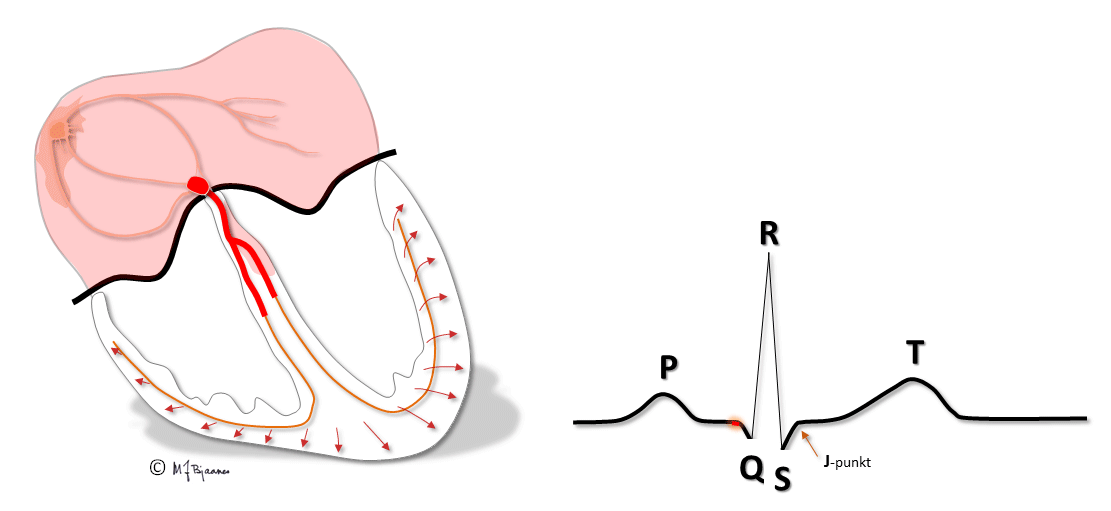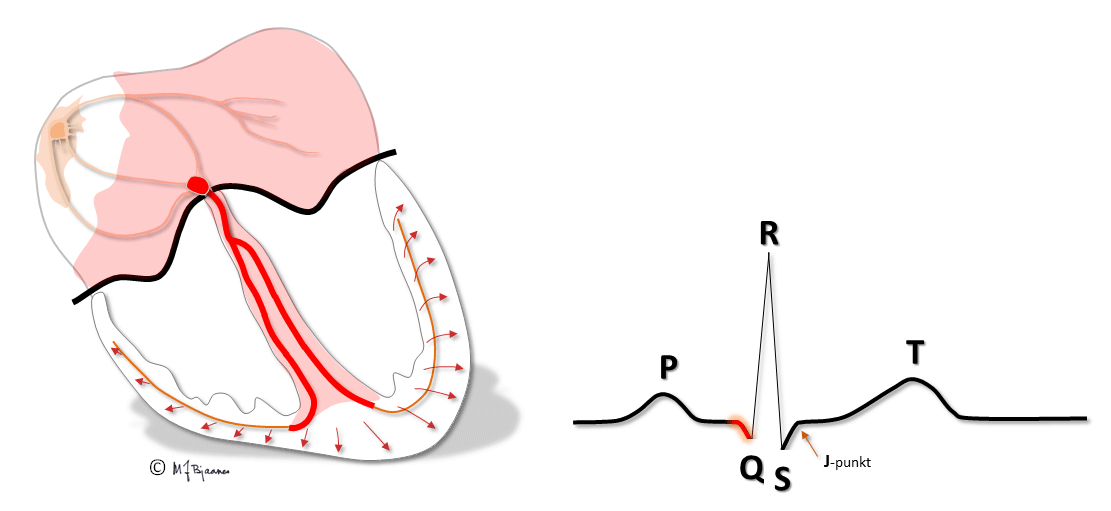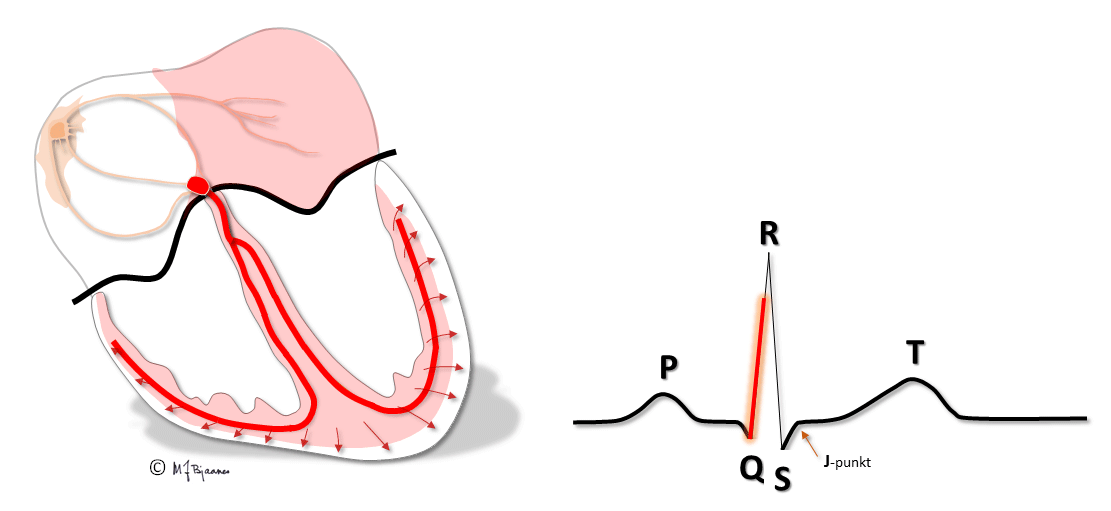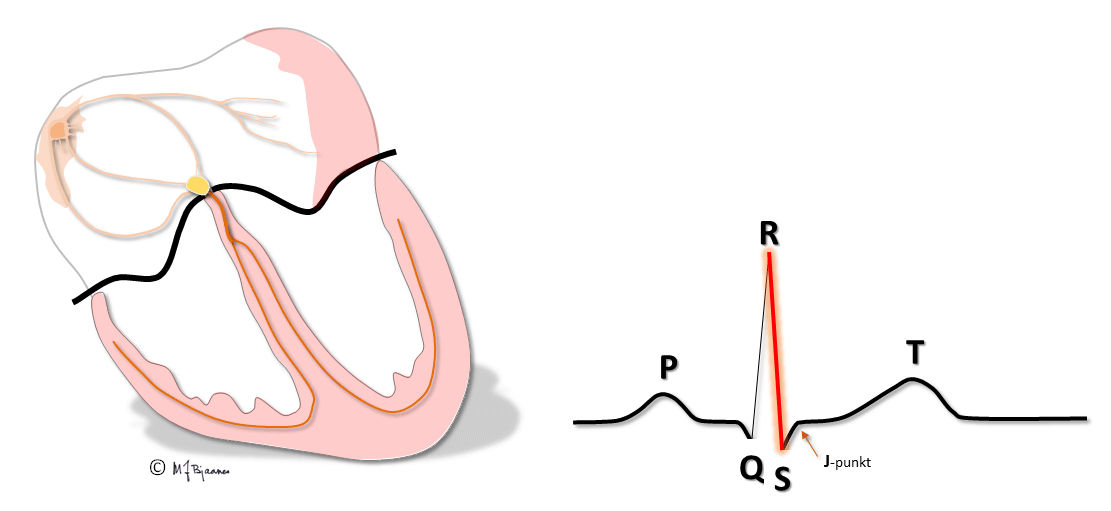
In endocardium the muscle cells have longer action potentials, whereas they are shorter in epicardium, due to different distribution of the various ion channels in the two layers. In epicardium, the transient outward potassium current, the Ito, is strong, causing a more distinct repolarization in phase I of the action potential.
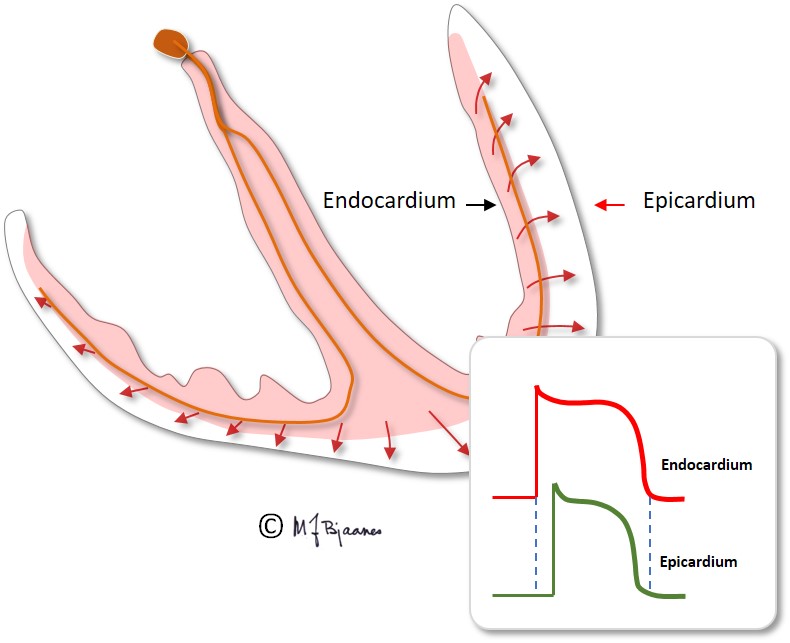
Therefore the electrical systole in epicardium finishes earlier than in endocardium, so repolarization starts close to the cardiac surface and spreads inward. A galvanometer outside the heart will then indicate a positive depolarization front, and the repolarization front with opposite charge, that moves away from the electrode, will then deflect similarly: the QRS of depolarization and the T-wave of repolarization normally point to the same direction, i.e. they are concordant.

The electrical activation of the cardiac chambers triggers a coordinated calcium mediated contraction of the chambers, following the action potential with a slight delay. This excitation-contraction coupling is both complicated and clinically important, but its presentation falls outside this course.

All simultaneous action potentials merge into the P-wave (the atria) and the QRS-T (ventricles):
Willem Einthoven named the waves of the ECG. Some think that the physiologists had used most of the letters in the alphabet, so P was the first one available. Others believe that he was inspired by Descartes, who used O (origo) in the center of his geometry, and then continued with P etc on the periphery.
Varighet maks 0,12 s; styrt av ledningshastigheten, av om atriebanen i taket mellom høyre og venstre atrie er intakt, og størrelsen på venstre atrium. Bredden måles fra første til siste avvik fra grunnlinjen i diastolen, som defineres som T-P-strekningen.
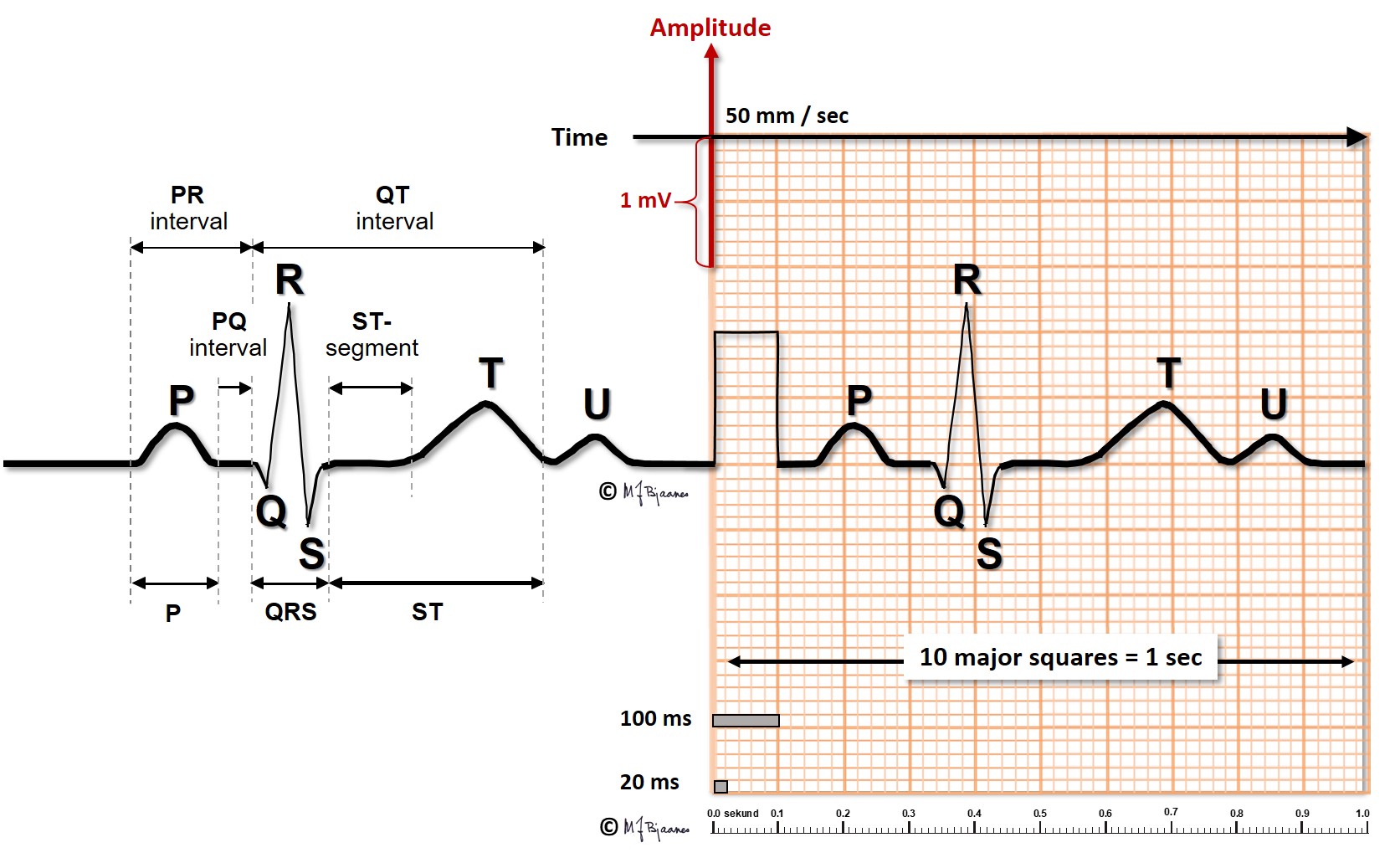
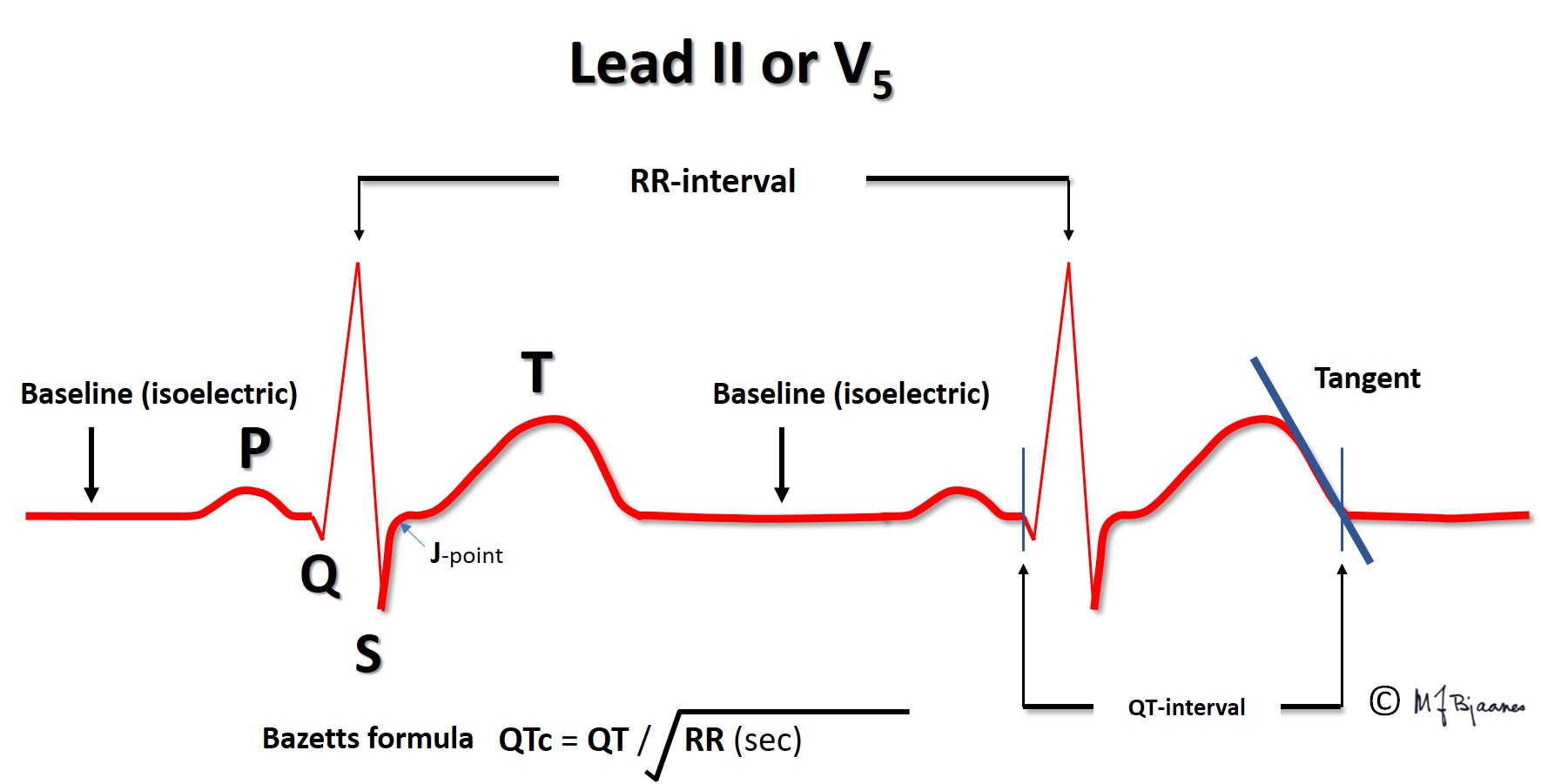
QRS reflects the depolarization of the ventricles. The impulses are conducted rapidly through bundle branches and purkinje fibres, resulting in peaked deflections. Q and S are both by definition, negative, and R and an R’ are positive (R’ is a positive deflection after an S). QRS terminates at the J point (J for junction), the point where the steep end of QRS suddenly flattens and marks the beginning of the ST segment.
The T wave represents the repolarization of the ventricles. The wave starts with a gentle slope, but has a steeper return to the baseline.
The QT-time reflects the duration of the systole, and is measured from the onset of the earliest Q wave to the end of the T wave, that is, the point where the tangent to the steepest downslope of the T wave crosses the baseline, preferentially on a lead close to the left ventricle (II, V5-V6). The action potentials are shortened when the heart beats faster, so the QT time is long in bradycardia and short in tachycardia. For this reason the measured QT time is «corrected» (QTc), usually by Bazett’s formula, that is
QTcorrected = QTmeasured / square root of the previous R-R interval (in seconds).
There are, however, alternative, more complicated formulas that may perform better.
At heart rate < 60 bpm, QTc < QTmeasured
At heart rate 60 bpm, QTc = QTmeasured
At heart rate > 60 bpm, QTc > QTmeasured
Occasionally one more wave is seen, on or after the T wave. This U wave represents the repolarization of cells with very long action potentials, either from purkinje fibres or from mid-myocardium. The U wave is not clinically important.
The baseline is the reference for measurement both of amplitudes and time intervals in the ECG. It is defined as the line between the end of the T wave and the onset of P, and is the most stable part of the ECG. To secure baseline stability during the recording, the patient should be supine and relaxed, and avoid deep breathing.