In this section, each segment of the ECG will be discussed, both what are normal findings, and how disturbances in- or outside the cell may influence the segment. In the next part (Part 3) we shall describe how pathophysiological disturbances impact upon the entire ECG.
The heart rate is normally defined by the sinus node. In adults this is normally 50-100 bpm at rest, but faster in children, during physical exercise or mental stress, during fever og anemia. The ability to increase heart rate contributes importantly to the increase in cardiac output during exercise; while the stroke volume merely can be doubled, the heart rate may rise by a factor of 3-4, to slightly above 200 bpm in the young, but maximal heart rate declines with aging.
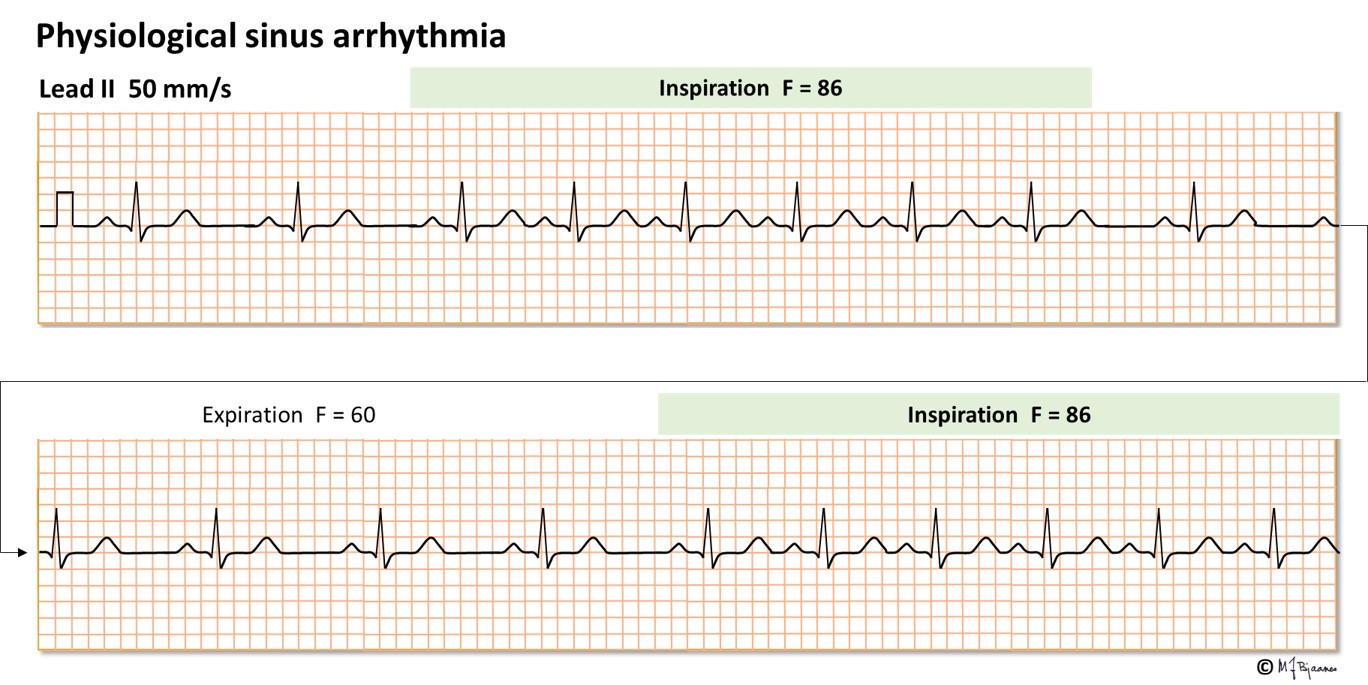
The normal heart rate varies with respiration; a vagus dependent effect. Such physiological respiratory sinus arrhythmia is often marked in children and the young, and is regarded as a healthy sign. The sinus node is an elongated structure, and the upper part contains the faster pacemaker cells.

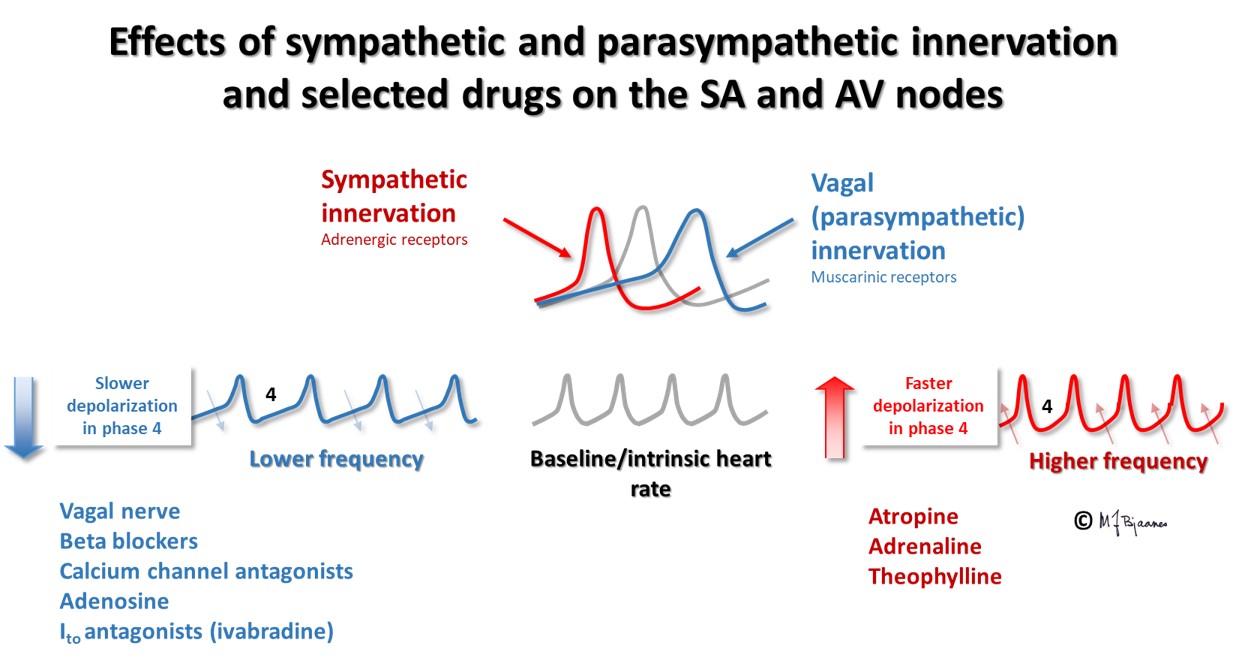
Many drugs influence the heart rate. The time to generation of the next action potential in a pacemaker cell depends on the spontaneous rise of the membrane potential in phase 4. The pacemaker current If contributes to this depolarization, and accordingly, when this channel is inhibited by the drug ivabradine, the heart rate is slower. Stimulation of the vagus nerve indirectly inhibits the pacemaker current, slowing the rise of the membrane potential in phase 4. In addition, the pacemaker cells have a potassium channel that is activated by acetylcholine from vagal nerve terminals. The resultant effect is that vagus stimulation reduces heart rate. A high vagal activity is seen in athletes at rest and when the old heart medicine digitalis is used, whereas if vagus is inhibited by atropine, heart rate increases. Sympathetic nerve or hormone stimulation of beta-adrenergic cell receptors increase the heart rate, whereas beta-adrenergic receptor blockers slows the heart rate, as also seen with calcium channel antagonists the reduces the spontaneous rise in membrane potential in phase 4. Also adenosine reduces the phase 4 rise by inhibiting the pacemaker current, in particular in the AV node, whereas aminophylline, a drug used in asthma, inhibits generation of adenosine and thus increases heart rate.
The P wave results from all atrial depolarization. The reason for the wave form is that the atria lack the specialized conduction system that provides the fast conduction that results in peaks in the QRS. Some atrial myocytes, however, are elongated and directed in parallel, justifying their name “atrial bundles”. Since longitudinal conduction in a muscle is much faster than transversal conduction, and the atria are smaller, the P wave is not broader than the QRS complex. In the roof of the atria an interatrial bundle connects the right and left atrium (Bachmann’s bundle).

The axis
High up in the right atrium we find the sinus node, where the depolarizing wave front is generated. The latter part of the P wave reflects activation of the left atrium. A normal P wave is smooth, rounded and terminates within 0.12 s. Activation usually starts high up in the right atrium, and spreads downward to the left. In the frontal plane the normal P wave axis is 0-75°, and the largest amplitude is 2.5 mm. The axis downward to the left results in a positive P wave in leads I and II.
As mentioned previously, the sinus node has downward extensions with pacemaker cells that gradually becomes slower. The vagus nerve is a stronger inhibitor high up, and hence, athletes usually have a slow resting heart rate, and often a P wave with an upward axis (negative P in aVF). When the lungs are hyperinflated, as in COPD with emphysema, the diaphragm is pushed down and flat, and the heart hangs like a droplet, resulting in a more vertical P axis. An abnormal P axis is also seen with extrasystoles that originate from pacemaker cells outside the sinus node, for instance in the pulmonary veins, the coronary sinus, caval veins or the atrial walls.


Amplitude
The amplitude of the P wave may be >2.5 mm in case the right atrium is enlarged or its wall is thickened (its position is close to the chest wall). This is seen when the right atrial work load is high. P pulmonale, a high, slightly peaked P wave, may be seen in pulmonary valve stenosis, pulmonary hypertension and occasionally in severe cases of COPD.

An enlarged left atrium will have little impact on the height of the P wave, since this atrium is located more posterior, away from the chest wall. If the interatrial roof bundle has reduced speed of conduction (due to inflammation or fibrosis), or the left atrium is enlarged (because of increased atrial afterload) the P wave becomes broader. Often a broad P wave (>0.12 s) may have two peaks, the first made by the right, the last one by the left atrium (a Mitral P wave, because it has an M shape, is typically found in mitral stenosis, since left atrial afterload then is high).

In failure of the left ventricle, its muscle is stiffer, and the required end-diastolic filling pressure increases. Such a diastolic dysfunction is often seen when the muscle becomes thicker, as in hypertension or aortic valve stenosis. The heart then rotates, and the enlarged left atrium is moved posteriorly (opposite to lead V1). In V1 we then see a broad P wave (>0.12 s), first positive (right atrial activation), but with a negative tail (left atrial activation) that is > 1 mm deep and > 40 ms long (“negative terminal P”), signifying left atrial overload.

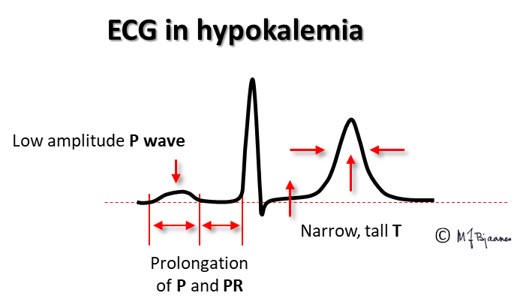
In hyperkalemia the resting membrane potential is less negative, and P wave amplitude and conduction velocity is reduced, giving lower and broader P waves.
The P wave represents atrial depolarization. Just like the ventricular depolarization (QRS) is followed by a repolarization wave (T), also the atria have their T (Ta). The axis of Ta is always opposite to the P wave. Usually the Ta is invisible, buried within the much larger QRS complex, but occasionally Ta is large, giving a bowl-shaped depression of QRS, a «junction depression (figure below).

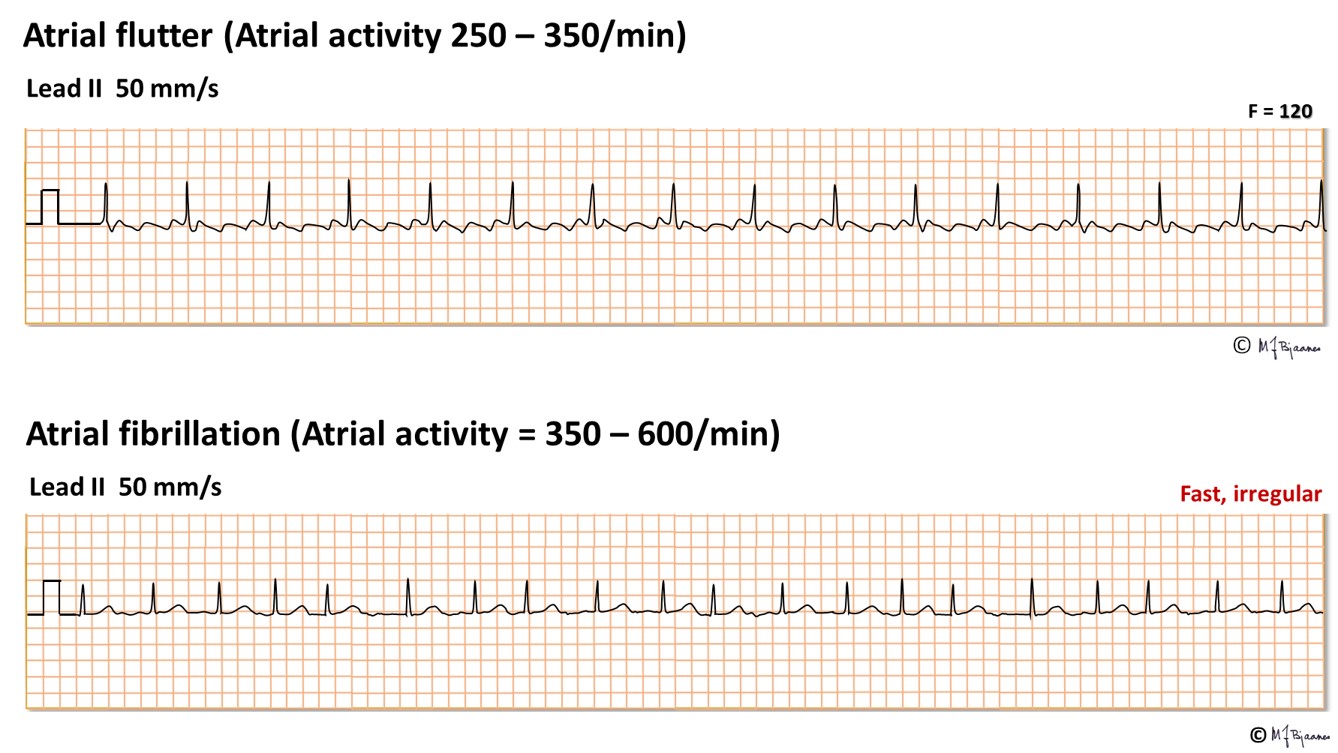
In atrial flutter, flutter waves at 300 bpm replace the P waves. If the atrial waves are even faster, as in atrial fibrillation, they show up as tiny wavelets, or even as a flat line.
This is the interval from the start of the P wave to the start of QRS, either to a q wave (if present), or an R (normally 0.12-0.21 s), reflecting the conduction time through the atria, the AV node, the bundle of His, the branches, fascicles and purkinje fibers. Most of the time is spent on passage through the AV node, and the delay here is useful, as it provides time for proper filling of the ventricles. The PQ interval shortens at faster heart rates.

A prolonged PQ interval (≥ 0.22 s) is called a first degree AV block. Actually, this is not a true block, but merely a delay. Vagal stimulation prolongs the PQ interval, and may even result in high grade AV block (more in Part 4 arrhythmias). This is probably the main mechanism for the effect of the old cardiac drug digitalis, that slows AV nodal conduction through influence upon the vagus nucleus in the brain stem. Also beta-adrenergic receptor blockers, non-dihydropyridine calcium channel inhibitors and the inhibitor of the «pacemaker current» If, ivabradine, all retard AV nodal impulse conduction.
A shortened PQ interval occurs when, in addition to the bundle of His, a conducting muscle fibre (an accessory bundle) passes through the annulus fibrosus, connecting an atrium and a ventricle. Such accessory bundles usually conduct faster than the AV node, and result in a premature slow QRS onset (preexcitation, Part 4, arrhythmias).
In pericardial infection/inflammation (pericarditis), the PQ segment is downsloping, probably because the myocytes that face the pericardium, are injured and get shorter action potentials moving the Ta forward.

QRS is the resultant of the depolarization front in time and space, and the spreading through the entire myocard. More correctly, it should be described by a vector, but the axis of the lead that shows the largest net QRS deflection, usually may be used as a crude estimate of the QRS axis. QRS deflections have sharp peaks: a Q wave when the first deflection is negative (downward). The R wave is the first positive (upward) peak, then a negative S wave may follow, and if another positive wave is seen, it is called an R’. Often large deflections are marked by capital case and smaller by letter case, for instance as qRs.
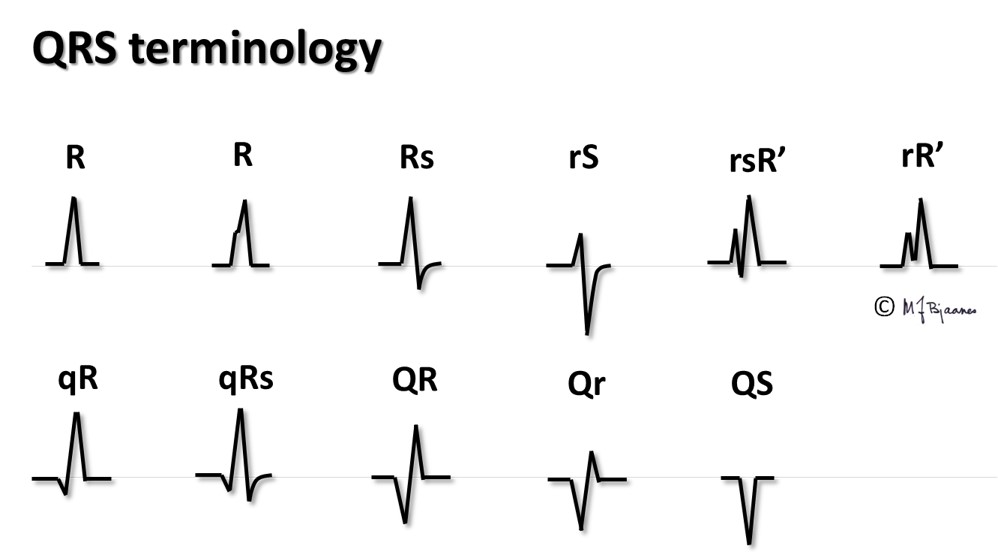
When the frontal plane leads are presented in Cabrera style (watch or compass format), the QRS axis can easily be estimated (see Part 1). The QRS axis is normally -30° to 105° in the frontal plane. The figure below is a reminder:

Right axis 90°–104° (often a normal variant, in particular in young persons)
Right axis deviation >105° (is seen in right ventricular hypertrophy and the rare left posterior hemiblock). Some interpretation programs regard any right axis as an axis deviation
Left axis <0° (normal)
Left axis deviation ≤-30° (often seen in left ventricular hypertrophy, but frequently remains unexplained)
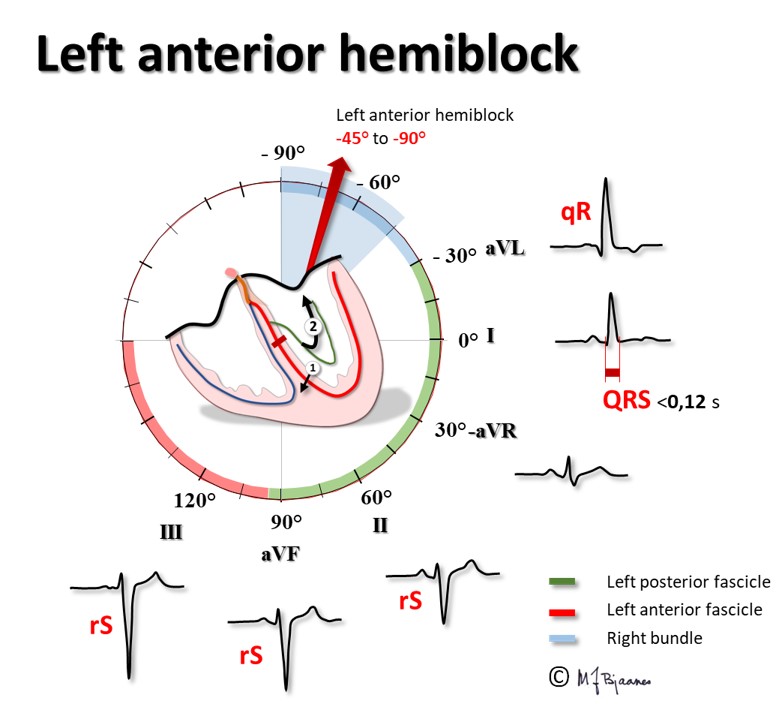
Left anterior hemiblock (or fascicular block) ≤-45° (often seen in left ventricular hypertrophy)
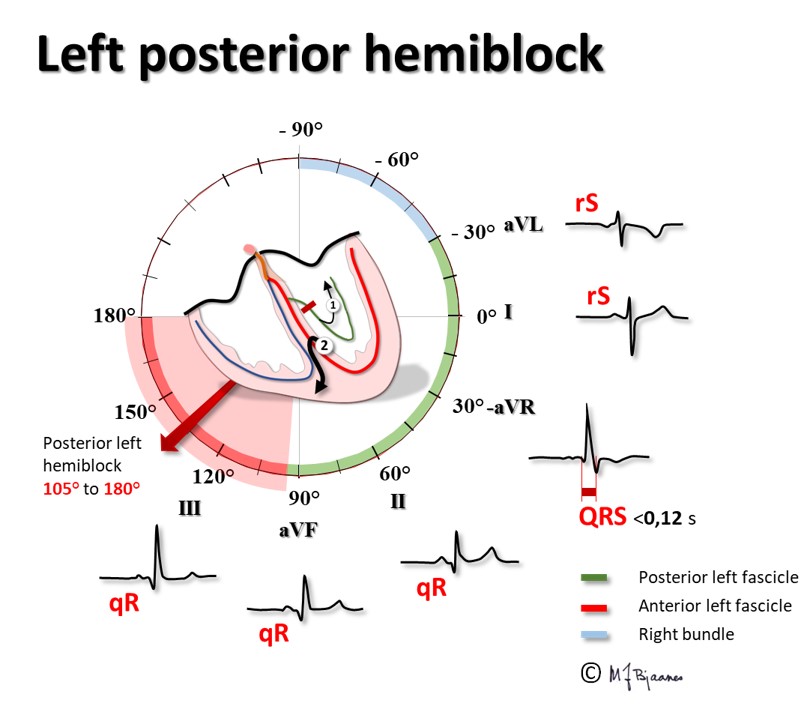
Left posterior hemiblock (or fascicular block) is rare. Normally, the first activation of the upper part of the interventricular septum originates from the left posterior fascicle, and the vector is directed anteriorly up to the right, creating the tiny r in lead V1 and the q wave in leads V6 and aVL. If this normal initial impulse does not appear, septal activation will start from lower down the left anterior fascicle; the vector will then point upwards, posterior and to the left, as that is where the larger muscle mass is located. We shall see an r wave in aVL and I, and a q wave in aVF and III), and the main activation of the left ventricle will come from the area near apex and move towards the right. The diagnosis of left posterior hemiblock thus requires 1. A QRS-axis of 90-180°, 2. an abnormal initial QRS vector, and 3. that the changes cannot be ascribed to pulmonary disease or to right ventricular pathology.

A Q wave is present when the first ventricular deflection is negative (downward). As mentioned above, the normal initial impulse comes from the left posterior fascicle, and is directed up toward the septum, anterior and to the right, giving a q wave in the leads that reflect the inferior wall (II, aVF and III) and the left side of the heart (I, V5-V6). aVR, however, «looks at» the inside of the ventricles. Since all activation spreads from endo- to epicardium, we find, as expected, a deep broad Q wave that fuses with the S wave; we have a QS wave). This implies that –aVR normally has no q wave, only a tall R. In V1 a q wave may occasionally be seen, but normally, definitively not in V2-V3. The duration of a normal q wave is <30 ms (0.03 seconds), with probably pathological values at 30-40 ms, and it is certainly pathological when 40+ ms, provided the amplitude is at least 25% of the R wave that follows. An isolated “pathological q wave” in lead III may, however, be accepted as normal. For the diagnosis of cell death (myocardial infarction), at least two neighbor leads with pathological q waves are convincing, whereas one pathological and one borderline q wave should raise strong suspicion that an infarction has occurred.
An r wave >40 ms duration in V1, with an R/S ratio above 1 and a concordant T wave, suggests loss of anterolateral electrical forces.
A paper speed of 50 mm/s facilitates the measurement of q wave duration; at 25 mm/s a probably pathological q wave (30+ ms) differs by only a quarter of a mm from a certainly pathological one (40+ ms).
These represent the main components of the QRS and determine the axis. The amplitudes reflect the combined effect of the electric activity of the heart, and the resistance of the tissues between the heart and the electrodes. Tissue is a better conductor than is blood, and a dilated failing ventricle that is filled with blood, results in smaller QRS amplitudes. A heart with much fibrosis or scar also has lower QRS amplitudes, as we also see in COPD, in particular in the presence of emphysema. In the latter case the heart is wrapped into isolating air filled lung tissue. The skin, however, has the highest resistance to conduction, hence a thickened dry skin results in low signal amplitudes.
QRS amplitudes are normallyCompared to females, QRS amplitudes are larger in men, in the young compared to the elderly, and in athletes compared to sedentary people. In hypertrophic cardiomyopathy we see large QRS amplitudes in addition to other ECG abnormalities. Hypertension and aortic valve stenosis are other examples of diseases where large QRS complexes are encountered.
Normally the lines describing the R and S are straight, without splitting (fragmentation). Usually fragmentation indicates heterogeneous conduction through myocard, most often due to fibrosis or scars. It is also associated with left ventricular hypertrophy, heart muscle diseases (cardiomyopathies) and myocardial infarctions. To be considered clinically important, the abnormality should be seen in (at least) two neighbor leads. Also the steepness of the deflections is of interest (dV/dt). In right ventricular hypertrophy, often an R>35 ms is seen in V1-V2, and, as expected, in left ventricular hypertrophy, delayed conduction is seen over apex (V5-V6) as a qR ≥50 ms.

The steepness of the deflection may also be helpful when broad complex QRS are analyzed: In bundle branch block the impulse propagates rapidly through the normal branch, resulting in a steep onset of QRS. The ventricle on the bundle branch side, is however, activated slowly outside the purkinje fibers, and the terminal 40 ms of the QRS complex is less steep than the onset. On the other hand, when the heart beat originates in a ventricle, the initial spread of impulses is slow, first to the myocytes nearby, then to the chambers. However, then impulses may enter the purkinje fibers and spread rapidly. Examples are a ventricular extrasystole and a preexcited beat (activation of myocardium by an accessory bundle that connects atrium and ventricle outside the bundle of His). This is illustrated (and exaggerated) in the figure below.

The QRS complex describes the depolarization of the ventricles. The time from the beginning to the end is normally up to 0.10 s (100 ms).
The bundle of His delivers impulses to the right and left bundle branches. The left branch divides into an anterior and a posterior fascicle, and sometimes into intermediary branches to the high septum. When the conduction through the left bundle branch is much slower than in the right bundle, or does not conduct at all, there is a left bundle branch block with a QRS complex lasting for ≥0.12 s, as the left ventricle now is activated from the right chamber, and transverse conduction takes time. The right ventricle is depolarized before the left one, and thus, all the terminal forces are directed to the left, giving a broad QRS with either one huge R or two R peaks in V5-V6. When there is a right bundle branch block, again, QRS is ≥0.12 s, and the terminal activation presents as an R’ in V1 (above the right ventricle). To memorize: the terminal activation (the R’) is ipsilateral; seen on the right side (V1) in right bundle branch block, and on the left side (V6) in left bundle branch block.
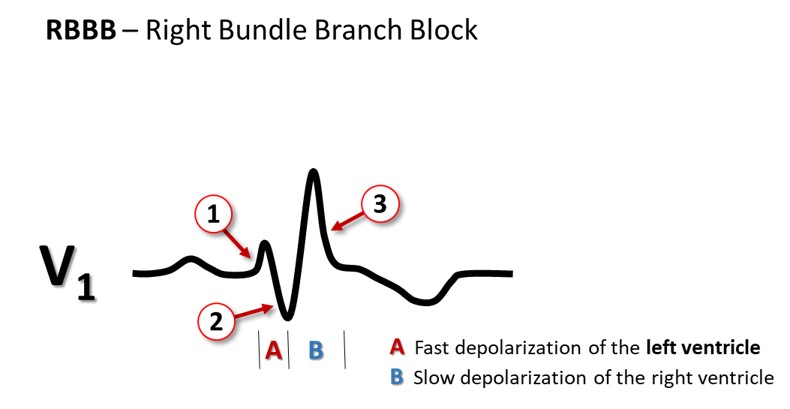
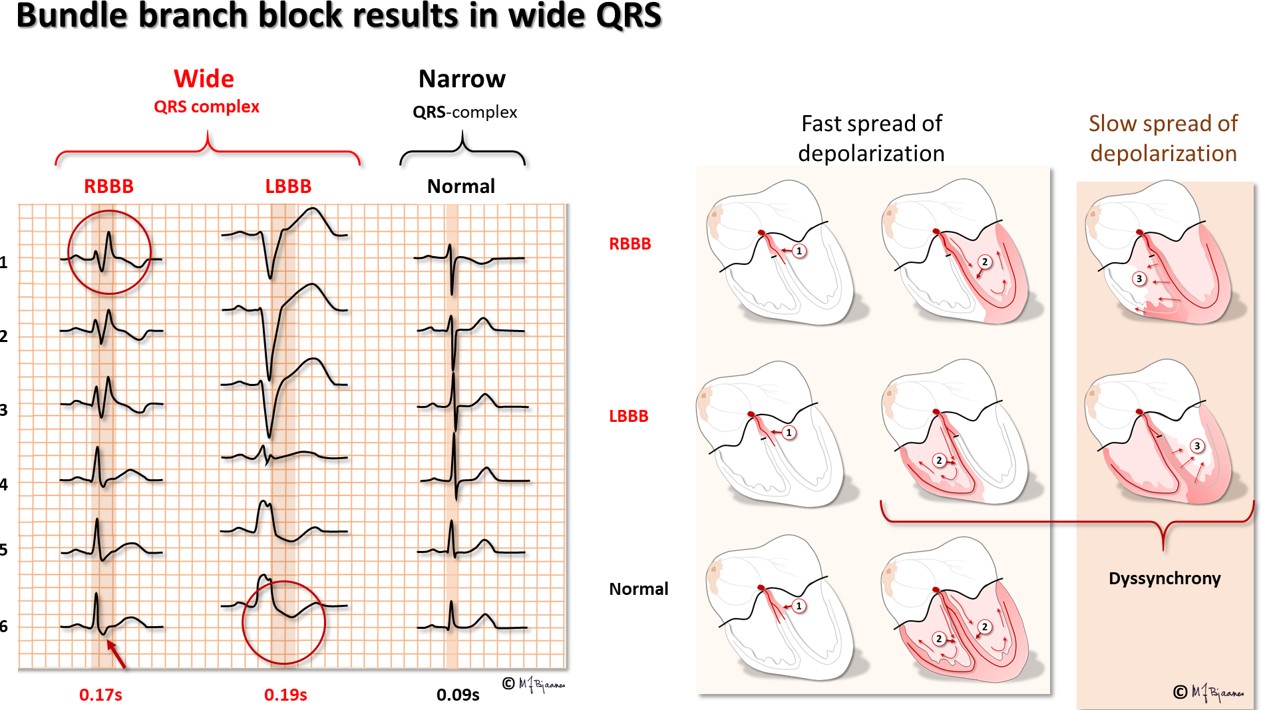
Incomplete right bundle branch block is frequently seen in otherwise normal hearts. Then QRS duration is 0.10 to <0.12 s, and V1 (and often also V2) show the RR’pattern. A block in the left anterior or posterior fascicle is called a hemiblock or fascicular block. Again the QRS is 0.10 to <0.12 s, and the QRS axis is abnormal (discussed later on).
A right bundle branch block has usually few clinical consequences (low pressure and low resistance in the pulmonary circulation). A left bundle branch block, however, weakens the cardiac work capacity much: The injury that has caused an LBBB is usually large since the left bundle branches early and the branches spread over a wide area. In addition, the septum will contract prior to the left ventricle, and bulge to the right when the left ventricle ejects blood.
Intraventricular conduction delay. A QRS with normal axis and morphology, but duration 0.10 to <0.12 s, represents a «slight intraventricular conduction delay», and a QRS ≥0,12 in a conducted beat is either right or left bundle branch (or preexcitation), or “considerable intraventricular delay”. A reduced conduction velocity (broad QRS complexes) is seen when myocardial potassium concentration is high (hyperkalemia, ischemia), and after an overdose of an antiarrhythmic drug that inhibits the sodium channels. The width of QRS also increases if the ventricles are enlarged (hypertrophy), in particular when there is myocardial fibrosis or scars.
Drugs that inhibit the sodium channels retard the depolarization (phase 0 of the action potential), and thereby reduce conduction velocity. The P wave slightly broadens, and the PQ interval and QRS width increases modestly. The ECG should be checked after start of treatment with such antiarrhythmic drugs, because too much widening of QRS may be a sign of overdose.

A (black) is a normal action potential, and B (blue) shows the action potential after intake of the sodium channel inhibitor flecainide, a drug that often is prescribed for prevention or termination of paroxysmal tachycardia. The slower action potential B reduces conduction velocity and prolongs the QRS (figures to the right).
If an atrial depolarization reaches the AV node too closely to the previous beat, the conduction system may be refractory, and the beat may be stopped (blocked), or the conduction system is partially refractory, and conduct slowly, prolonging the PQ interval and widening the QRS, perhaps with a hemiblock or a bundle branch block. This «aberrant» conduction of supraventricular beats usually occurs for an early supraventricular extrasystole or at a high heart rate. Broad, aberrantly conducted innocent beats must not be confused with potentially dangerous beats that are broad because they originate in a ventricle. A rule of the thumb is that a narrow QRS is supraventricular and a broad QRS ventricular, until otherwise proven.
| Aberrant conduction | Ventricular beat | |
|---|---|---|
| Relation to P | P wave before each QRS or atrial flutter/fibrillation | No P wave before QRS Occasionally a retrograde P wave after the QRS |
| QRS duration | Usually 0.12-0.15 sec. | Usually ≥0.15 sec. |
| QRS morphology | Looks like a bundle branch block Duration varies with distance to previous beat, with steep onset and slow termination | Different from a bundle branch block, may fuse with a normal beat, have a slow QRS onset and a steeper termination |
The J point denotes the relatively sharp transition from the QRS complex to the ST segment, coinciding with the transient outward repolarizing potassium current Ito (phase I of the action potential).

Usually the J point is positioned on the baseline, but in «early repolarization» it may be elevated. This is a normal variant, often seen in young well trained men. Ito is stimulated by vagus (J point elevation). The chest leads V2 and V3 are close to the septum, which is a special area with a surface facing the chest and two endocardial sides. Normally, the J point here is elevated, in general up to 2 mm, and in male athletes, up to 3 mm is permitted.
An upward concave ST elevation is usually innocent, but when an elevated or depressed ST segment is horizontal or has a downward slope, pathology is usually present.
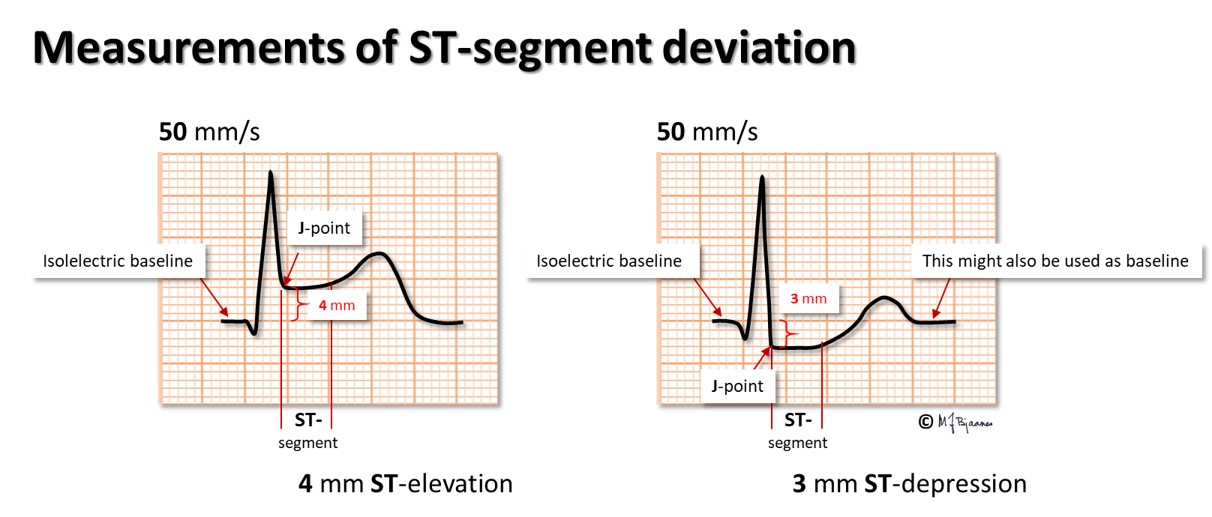
In ischemia the ST segment deviates from the baseline for at least two reasons:


In remote ischemia, there are healthy myocytes between the ischemic area and the electrode. The diastolic current of injury is now directed toward the electrode, and the baseline is elevated. Then it looks as if the fixed QRST is lowered. Such an ST depression is typically seen in angina pectoris, where the ischemia is mainly subendocardial, as the inner part of the myocardium is far away from the epicardial coronary arteries, and the tissue pressure here is highest. We also encounter a reciprocal ST depression in remote ischemia, for instance when the diastolic current of injury from posterior wall myocardial infarction is directed toward the anterior wall, elevating the baseline there, and seemingly show an ST depression in the precordial leads.

In ST-elevation myocardial infarction, STEMI, the ST elevation is measured from the level at the onset of the QRS complex to the J point, the take-off for the ST segment. The sum of ST elevation in all the 12 ECG leads, early in the course of a STEMI, gives a fair estimate of the heart muscle mass that is at risk.
During an exercise ECG test, we often see that a J point moves downward from its position at rest, followed by a rapidly ascending ST segment. This is a normal response to the sympathetic stimulation of the heart. Hence, ST depression is not assessed at the J point, but 80 ms later, and measured as the deviation from baseline, defined as the stable T-P segment. In tachycardia, as during an exercise test, the QT interval is shortened, and the ST deviation is measured earlier, 60 ms after the J point. A horizontal or downsloping ST segment is usually pathological.
In earlier days the fox glove extract digitalis was frequently prescribed, and its synthetic version is still used by patients with rapidly conducted atrial fibrillation combined with heart failure. This drug inhibits the Na+/K+-ATPase (that expels 3 Na+ in exchange for the entry of 2 K+). The resting membrane potential thus becomes less negative on digitalis treatment, causing a less steep rise in phase 0 of the action potential. More intracellular sodium stimulates the Na+/Ca2+ exchanger, and the increased intracellular calcium concentration results in increased contractility and a shortened phase 2. As the phase 1 transient outward potassium current is stronger in epicardial compared to endocardial cells, the transmural difference in action potential phase 2 is augmented when action potential duration is shortened, and the difference in potential is reflected as scoop shaped ST depression, that may be hard to distinguish from ischemia.

The T wave represents repolarization of the ventricles (the phase 3 of the action potential), when potassium channels open for ion extrusion. The wave starts slowly and ends steeper. Normally, the T wave has the same direction (axis) as its QRS. Intuitively this is illogical because we expect that discharge and recharge should have opposite vector direction. The explanation is, however, that the entire subendocardium is activated almost simultaneously from the purkinje fibers, and the front of depolarization spreads outward to the epicardium. As the duration of subendocardial action potentials is longer than those of the epicardium, repolarization spreads inwards. An opposite charge in an opposite direction results in ECG deflections with near similar QRS and T wave axes (QRS and T wave are concordant), though there are some exceptions: The right ventricular wall is thin compared to the left side, and the interventricular septum has two endocardial sides. This may explain the usual (and normal) finding of an upright T wave in leads V1 and V2, in spite of the negative S wave that dominates the QRS complex. In females, a discordant T wave is permitted even in V3, provided there are negative T waves also in V1-V2. But elsewhere, discordant T waves in neighbor leads are regarded pathological.
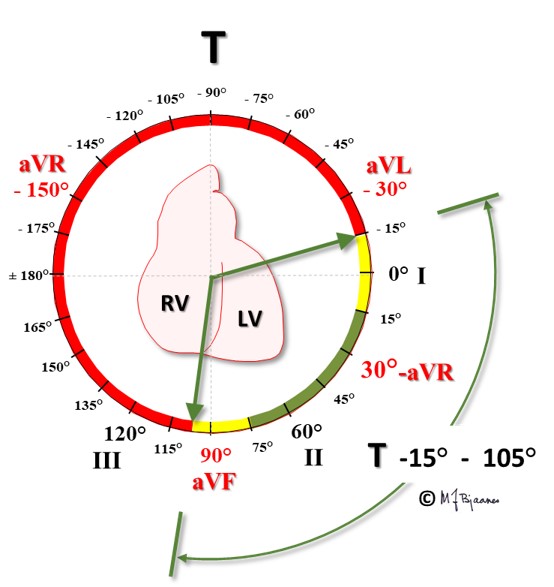
T wave axis: green is normal, yellow is borderline, and the red zone denotes pathology.

In hyperkalemia the resting membrane potential is less negative, and the repolarization needs a shorter time; we see a narrow, high peaked T wave. In hypokalemia the cells must regain a more negative membrane potential, and the T waves become broad and flat.

The QT time reflects the duration of systole, that is the time from the first to the last depolarization in the ventricles. Thus, it is affected by both the duration of the action potential and the time from the first to the last depolarization (i.e. the QRS width). If the systolic phase 2 is prolonged, calcium channels may be reactivated. When neighbor cells have reached different stages of the action potential, the voltage gradient between them may open sodium channels, triggering an afterpotentials that may cause an extra (triggered) beat, or even a series of rapid beats that may cause syncope or in worst case, sudden cardiac death. For this reason it is important to check the QT time in syncopal patients, in the relatives to patients who have suffered from a sudden, unexpected cardiac arrest, and after prescription of drugs that are known to prolong the QT interval. (More on Long QT syndromes (LQTS) in Part 4, Arrhythmias).
QT interval is measured from the onset of the QRS complex to the end of the T wave in the same lead, defined as the point when the tangent to the steepest T wave deflection crosses the baseline.

The action potentials are long at slow heart rates and shorten at faster rates. The measured QT interval must therefore be standardized (corrected, QTc) to a calculated interval at the rate of 60 bpm. Several formulas may be used for this, and the formula of Bazett is the usual, simple one (although not the best one):
QTc= QTmeasured/ square root of the preceding R to R interval, measured in seconds.
Bazett’s formula works well in the range 50-100 bpm, but performs poorly in brady- or tachycardia.
At heart rates <60 bpm, QTc < QTmeasured
At heart rate 60 bpm, QTc = QTmeasured
At heart rates >60 bpm, QTc > QTmeasured

Females have slighly longer QTc than men: normal maximum is 0.46 s, versus 0.44 for males (limits are not universally accepted). A rule of thumb is that QT should be shorter that half the preceding R-R interval. Only when QTc >0.50, the risk of arrhythmias increases steeply.
Extracellular potassium- and calcium concentrations influence the QT time.
Phase 2 is prolonged when s-Ca2+ is lower than normal, whereas hypercalcemia shortens the QT time. Magnesium (Mg2+) is in part a physiological calcium antagonist, and is used to treat arrhythmias caused by a long QT interval, in spite of the fact that Mg2+ per se may prolong the QT interval. In hyperkalemia, the resting membrane potential is less negative, and is restored faster than normal in phase III, whereas in hypokalemia, more time is needed to reach the more negative resting membrane potential, and the QT time increases.
This wave is clinically unimportant, but it may interfere with the measurement of the QT interval when fusing with the T wave. In such cases QT time is measured to the end of the U wave, whereas it is not included when the U wave is separate.
The unexperienced ECG interpreter MUST use a systematic approach, whereas an expert often may rely on pattern recognition. However, when anything does not fit completely with the expected pattern, one must revert to the systematic approach.
It is recommended first to check some basic parameters before studying details. When QRS is wide (>0.12 s), the common rules for QRS and T wave morphology are no longer valid.
Systematic analysis
When there are more than one type of complexes, each one should be analyzed separately.
You should look at:
The first step is strictly descriptive. The second is the technical interpretation, and the third, interpreting steps 1 and 2 in the relevant clinical context. An example follows:
Waves
Intervals and the baseline